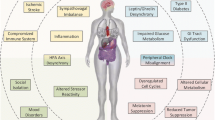
Abstract
Chronic stress activates the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis to aggravates tumorigenesis and development. Although the importance of SNS and HPA in maintaining homeostasis has already attracted much attention, there is still a lot remained unknown about the molecular mechanisms by which chronic stress influence the occurrence and development of tumor. While some researches have already concluded the mechanisms underlying the effect of chronic stress on tumor, complicated processes of tumor progression resulted in effects of chronic stress on various stages of tumor remains elusive. In this reviews we concluded recent research progresses of chronic stress and its effects on premalignancy, tumorigenesis and tumor development, we comprehensively summarized the molecular mechanisms in between. And we highlight the available treatments and potential therapies for stressed patients with tumor.



Similar content being viewed by others
References
Chida Y, Sudo N, Takaki A, Kubo C (2005) The hepatic sympathetic nerve plays a critical role in preventing Fas induced liver injury in mice. Gut 54:994–1002. https://doi.org/10.1136/gut.2004.058818
Wang K, Zhao XH, Liu J, Zhang R, Li JP (2020) Nervous system and gastric cancer. Biochim Biophys Acta Rev Cancer 1873:188313. https://doi.org/10.1016/j.bbcan.2019.188313
Chen R et al (2019) Prostate cancer risk prediction models in Eastern Asian populations: current status, racial difference, and future directions. Asian J Androl 21:1–4. https://doi.org/10.4103/aja.aja
Mravec B, Tibensky M, Horvathova L (2020) Stress and cancer. Part I: mechanisms mediating the effect of stressors on cancer. J Neuroimmunol 346:577311. https://doi.org/10.1016/j.jneuroim.2020.577311
Article R (2008) Invited minireview: Stress-induced remodeling of lymphoid innervation. Bone 23:1–7. https://doi.org/10.1016/j.bbi.2007.06.011.Invited
Li H et al (2013) Toll-like receptor 9 is required for chronic stress-induced immune suppression. NeuroImmunoModulation 21:1–7. https://doi.org/10.1159/000354610
Klopack ET (2022) Social stressors associated with age-related T lymphocyte percentages in older US adults : Evidence from the US Health and Retirement Study. 1–5 https://doi.org/10.1073/pnas.2202780119/-/DCSupplemental.Published
Jung S et al (2020) Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy 16:512–530. https://doi.org/10.1080/15548627.2019.1630222
Jones R (2014) Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Bone 23:1–7. https://doi.org/10.1002/jbmr.1562.Multipotent
Sloan EK et al (2007) Social stress enhances sympathetic innervation of primate lymph nodes: Mechanisms and implications for viral pathogenesis. J Neurosci 27:8857–8865. https://doi.org/10.1523/JNEUROSCI.1247-07.2007
Kantarjian H, Garcia-Manero G, Yang HSQKSODT (2005) Social temperament and lymph node innervation. Bone 23:1–7. https://doi.org/10.1016/j.bbi.2007.10.010.Social
Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12:31–46. https://doi.org/10.1158/2159-8290.CD-21-1059
Androulidaki A et al (2009) Corticotropin releasing factor promotes breast cancer cell motility and invasiveness. Mol Cancer 8:1–12. https://doi.org/10.1186/1476-4598-8-30
Shi M et al (2011) The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat 125:351–362. https://doi.org/10.1007/s10549-010-0822-2
Saul AN et al (2005) Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 97:1760–1767. https://doi.org/10.1093/jnci/dji401
Parker J et al (2004) Chronic stress accelerates ultraviolet-induced cutaneous carcinogenesis. J Am Acad Dermatol 51:919–922. https://doi.org/10.1016/j.jaad.2004.08.042
Sloan EK et al (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70:7042–7052. https://doi.org/10.1158/0008-5472.CAN-10-0522
Zhi X et al (2019) Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int J Oncol 54:1625–1638. https://doi.org/10.3892/ijo.2019.4753
Cui B et al (2019) Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest 129:1030–1046. https://doi.org/10.1172/JCI121685
Hasegawa H, Saiki I (2002) Psychosocial stress augments tumor development through β-adrenergic activation in mice. Jpn J Cancer Res 93:729–735. https://doi.org/10.1111/j.1349-7006.2002.tb01313.x
Biegler KA, Anderson AKL, Wenzel LB, Osann K, Nelson EL (2012) Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: Implications for cancer prevention. Cancer Prev Res 5:1173–1182. https://doi.org/10.1158/1940-6207.CAPR-12-0008
James GD, Van Berge-Landry H, Valdimarsdottir HB, Montgomery GH, Bovbjerg DH (2004) Urinary catecholamine levels in daily life are elevated in women at familial risk of breast cancer. Psychoneuroendocrinology 29:831–838. https://doi.org/10.1016/S0306-4530(03)00150-1
Putu D, Shoveller J, Montaner J, Feng C, Nicoletti R, Shannon K (2016) Neural regulation of hematopoiesis, inflammation and cancer. Physiol Behav 176:139–148. https://doi.org/10.1016/j.neuron.2015.01.026.Neural
Nelson EL et al (2008) Stress, immunity, and cervical cancer: Biobehavioral outcomes of a randomized clinical trail. Clin Cancer Res 14:2111–2118. https://doi.org/10.1158/1078-0432.CCR-07-1632
Chang CH, Chen SJ, Liu CY (2015) Risk of developing depressive disorders following hepatocellular carcinoma: a nationwide population-based study. PLoS ONE 10:1–10. https://doi.org/10.1371/journal.pone.0135417
Alonso C et al (2008) Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology 135:163–172. https://doi.org/10.1053/j.gastro.2008.03.036
Zhang X et al (2019) Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. https://doi.org/10.1038/s41419-019-2030-2
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K (2011) Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 29:2635–2644. https://doi.org/10.1200/JCO.2010.33.5422
Chang A et al (2016) β2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav Immun 57:106–115. https://doi.org/10.1016/j.bbi.2016.06.011
Armaiz-Pena GN et al (2013) Src activation by adrenoreceptors is a key switch for tumour metastasis. Nat Commun. https://doi.org/10.1038/ncomms2413
Lin Q et al (2013) Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS ONE. https://doi.org/10.1371/journal.pone.0061435
Roy S et al (2018) The role of p38 MAPK pathway in p53 compromised state and telomere mediated DNA damage response. Mutat Res Genet Toxicol Environ Mutagen 836:89–97. https://doi.org/10.1016/j.mrgentox.2018.05.018
Flint MS, Bovbjerg DH (2012) DNA damage as a result of psychological stress: implications for breast cancer. Breast Cancer Res 14:3–5. https://doi.org/10.1186/bcr3189
Feng Z et al (2012) Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci USA 109:7013–7018. https://doi.org/10.1073/pnas.1203930109
Reeder A et al (2015) Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer 112:1461–1470. https://doi.org/10.1038/bjc.2015.133
Thomas M et al (2018) Impaired PARP activity in response to the β-adrenergic receptor agonist isoproterenol. Toxicol Vitr 50:29–39. https://doi.org/10.1016/j.tiv.2018.02.001
Baritaki S, de Bree E, Chatzaki E, Pothoulakis C (2019) Chronic stress, inflammation, and colon cancer: a CRH system-driven molecular crosstalk. J Clin Med 8:1669. https://doi.org/10.3390/jcm8101669
Wei L et al (2019) Chronic unpredictable mild stress in rats induces colonic inflammation. Front Physiol 10:1–12. https://doi.org/10.3389/fphys.2019.01228
Gao X et al (2018) Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA 115:E2960–E2969. https://doi.org/10.1073/pnas.1720696115
Wu W et al (2014) Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut 63:1883–1892. https://doi.org/10.1136/gutjnl-2013-306083
Melinder C, Hiyoshi A, Fall K, Halfvarson J, Montgomery S (2017) Stress resilience and the risk of inflammatory bowel disease: a cohort study of men living in Sweden. BMJ Open 7:1–8. https://doi.org/10.1136/bmjopen-2016-014315
Mawdsley JE, Rampton DS (2005) Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 54:1481–1491. https://doi.org/10.1136/gut.2005.064261
Salvo-Romero E et al (2020) Overexpression of corticotropin-releasing factor in intestinal mucosal eosinophils is associated with clinical severity in Diarrhea-Predominant Irritable Bowel Syndrome. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-77176-x
March-Luján VA et al (2021) Impact of BMGIM music therapy on emotional state in patients with inflammatory bowel disease: a randomized controlled trial. J Clin Med 10:1591. https://doi.org/10.3390/jcm10081591
Han Y et al (2020) Hydrogen sulfide-mediated resistance against water avoidance stress-induced gastritis by maintenance of gastric microbial homeostasis. Microbiologyopen 9:1–18. https://doi.org/10.1002/mbo3.951
Takada S et al (2021) Stress can attenuate hepatic lipid accumulation via elevation of hepatic β-muricholic acid levels in mice with nonalcoholic steatohepatitis. Lab Investig 101:193–203. https://doi.org/10.1038/s41374-020-00509-x
Yang X et al (2014) Chronic restraint stress decreases the repair potential from mesenchymal stem cells on liver injury by inhibiting TGF-β1 generation. Cell Death Dis 5:1–10. https://doi.org/10.1038/cddis.2014.257
Nakade Y, Yoneda M, Nakamura K, Makino I, Terano A (2002) Involvement of endogenous CRF in carbon tetrachloride-induced acute liver injury in rats. Am J Physiol Regul Integr Comp Physiol 282:1782–1788. https://doi.org/10.1152/ajpregu.00514.2001
Oben JA et al (2004) Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 53:438–445. https://doi.org/10.1136/gut.2003.026658
Dubuisson L et al (2002) Inhibition of rat liver fibrogenesis through noradrenergic antagonism. Hepatology 35:325–331. https://doi.org/10.1053/jhep.2002.31166
Article R (2008) Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Bone 23:1–7. https://doi.org/10.1053/j.gastro.2013.01.002.Role
Zhao W et al (2011) Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer 129:2651–2661. https://doi.org/10.1002/ijc.25920
Nasrullah M (2018) (2016) Regulation of non small-cell lung cancer stem cell like cells by neurotransmitters and opioid peptides. Physiol Behav 176:139–148. https://doi.org/10.1038/nn.4087.Stress
Bruffaerts R, Mortier PH, Kiekens G, Auerbach RP, Cuijpers P, Demyttenaere K, Green JG, Nock MK, Kessler RC (2017) Nicotine induces self-renewal of pancreatic cancer stem cells via neurotransmitter-driven activation of sonic hedgehog signaling. Physiol Behav 176:139–148. https://doi.org/10.1016/j.ejca.2015.10.003.Nicotine
Lin XH et al (2020) Norepinephrine-stimulated HSCs secrete sFRP1 to promote HCC progression following chronic stress via augmentation of a Wnt16B/β-catenin positive feedback loop. J Exp Clin Cancer Res 39:1–17. https://doi.org/10.1186/s13046-020-01568-0
Zhang B et al (2020) The stress hormone norepinephrine promotes tumor progression through β2-adrenoreceptors in oral cancer. Arch Oral Biol. https://doi.org/10.1016/j.archoralbio.2020.104712
Sorrentino G et al (2017) Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. https://doi.org/10.1038/ncomms14073
Kim SL, Choi HS, Kim JH, Lee DS (2020) The antiasthma medication ciclesonide suppresses breast cancer stem cells through inhibition of the glucocorticoid receptor signaling-dependent YAP pathway. Molecules. https://doi.org/10.3390/molecules25246028
He L et al (2019) Glucocorticoid receptor signaling activates TEAD4 to promote breast cancer progression. Cancer Res 79:4399–4411. https://doi.org/10.1158/0008-5472.CAN-19-0012
Zhu Z et al (2021) Stress-related hormone reduces autophagy through the regulation of phosphatidylethanolamine in breast cancer cells. Ann Transl Med 9:149–149. https://doi.org/10.21037/atm-20-8176
Sood AK et al (2010) Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120:1515–1523. https://doi.org/10.1172/JCI40802
Hassan S et al (2013) Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 123:874–886. https://doi.org/10.1172/JCI63324
Zhang D et al (2011) β 2-adrenoceptor blockage induces G 1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol Cancer 10:1–9. https://doi.org/10.1186/1476-4598-10-146
Nasrullah M (2018) (2016) Chronic variable stress activates hematopoietic stem cells. Physiol Behav 176:139–148. https://doi.org/10.1038/nm.3589.Chronic
Dutta P et al (2012) Myocardial infarction accelerates atherosclerosis. Nature 487:325–329. https://doi.org/10.1038/nature11260
Méndez-Ferrer S, Lucas D, Battista M, Frenette PS (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452:442–447. https://doi.org/10.1038/nature06685
Article R (2008) Circadian control of the immune system. Bone 23:1–7. https://doi.org/10.1038/nri3386.Circadian
Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang J, Zhang D, Hashimoto D, Merad MPSF (2013) Adrenergic nerves govern circadian leukocyte. Immunity 37:290–301. https://doi.org/10.1016/j.immuni.2012.05.021.ADRENERGIC
Niraula A, Wang Y, Godbout JP, Sheridan JF (2018) Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J Neurosci 38:2328–2340. https://doi.org/10.1523/JNEUROSCI.2568-17.2018
Powell ND et al (2013) Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 110:16574–16579. https://doi.org/10.1073/pnas.1310655110
Irwin MR, Cole SW (2011) Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 11:625–632. https://doi.org/10.1038/nri3042
Bondar T, Medzhitov R (2013) The origins of tumor-promoting inflammation. CCELL 24:143–144. https://doi.org/10.1016/j.ccr.2013.07.016
Hodge DR, Hurt EM, Farrar WL (2005) The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41:2502–2512. https://doi.org/10.1016/j.ejca.2005.08.016
Nilsson MB et al (2007) Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem 282:29919–29926. https://doi.org/10.1074/jbc.M611539200
Shahzad MMK et al (2010) Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem 285:35462–35470. https://doi.org/10.1074/jbc.M110.109579
Armaiz-Pena GN et al (2015) Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 6:4266–4273. https://doi.org/10.18632/oncotarget.2887
M.L. Hanke et al (2013) Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Bone 23:1–7. https://doi.org/10.1016/j.bbi.2012.07.011.Beta
Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylande BL (2018) Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol 9:1–15. https://doi.org/10.3389/fimmu.2018.00164
Sommershof A, Scheuermann L, Koerner J, Groettrup M (2017) Chronic stress suppresses anti-tumor TCD8+ responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav Immun 65:140–149. https://doi.org/10.1016/j.bbi.2017.04.021
Inbar S et al (2011) Do stress responses promote leukemia progression? an animal study suggesting a role for epinephrine and prostaglandin-e2 through reduced nk activity. PLoS ONE. https://doi.org/10.1371/journal.pone.0019246
Zhao Y et al (2019) Depression promotes hepatocellular carcinoma progression through a glucocorticoid-mediated upregulation of PD-1 expression in tumor-infiltrating NK cells. Carcinogenesis 40:1132–1141. https://doi.org/10.1093/carcin/bgz017
Rosenne E (2014) In vivo suppression of NK cell cytotoxicity by stress and surgery in F344 rats: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun 23:1–7. https://doi.org/10.1016/j.bbi.2013.12.007.In
Ayroldi E, Cannarile L, Adorisio S, Delfino DV, Riccardi C (2018) Role of endogenous glucocorticoids in cancer in the elderly. Int J Mol Sci. https://doi.org/10.3390/ijms19123774
Zhao J et al (2015) TLR2 involved in naive CD4+ T cells rescues stress-induced immune suppression by regulating Th1/Th2 and Th17. NeuroImmunoModulation 22:328–336. https://doi.org/10.1159/000371468
Muthuswamy R et al (2017) Epinephrine promotes COX-2-dependent immune suppression in myeloid cells and cancer tissues. Brain Behav Immun 62:78–86. https://doi.org/10.1016/j.bbi.2017.02.008
Lutgendorf SK et al (2014) Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. 131: 319–335 https://doi.org/10.1016/j.bbi.2007.12.012.Depressed
Mohammadpour H et al (2019) Β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129:5537–5552. https://doi.org/10.1172/JCI129502
Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE (2011) Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol 270:80–87. https://doi.org/10.1016/j.cellimm.2011.04.003
Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S (2009) Catecholamines regulate tumor angiogenesis. Cancer Res 69:3727–3730. https://doi.org/10.1158/0008-5472.CAN-08-4289
Thaker PH et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–944. https://doi.org/10.1038/nm1447
Xie H et al (2015) Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol 51:991–997. https://doi.org/10.1016/j.oraloncology.2015.08.007
Aslam N, Nadeem K, Noreen RJAC (2015) Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Abeloff’s Clin Oncol 5(8):938–944
Liu GX et al (2016) Isoprenaline induces periostin expression in gastric cancer. Yonsei Med J 57:557–564. https://doi.org/10.3349/ymj.2016.57.3.557
Madel MB, Elefteriou F (2021) Mechanisms supporting the use of beta-blockers for the management of breast cancer bone metastasis. Cancers (Basel) 13:1–18. https://doi.org/10.3390/cancers13122887
Haldar R et al (2018) Perioperative inhibition of β-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun 73:294–309. https://doi.org/10.1016/j.bbi.2018.05.014
Moretti S et al (2013) Β-Adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Investig 93:279–290. https://doi.org/10.1038/labinvest.2012.175
Pan C et al (2021) Depression accelerates gastric cancer invasion and metastasis by inducing a neuroendocrine phenotype via the catecholamine/β2-AR/MACC1 axis. Cancer Commun. https://doi.org/10.1002/cac2.12198
Landen CN et al (2007) Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res 67:10389–10396. https://doi.org/10.1158/0008-5472.CAN-07-0858
Bruffaerts R, Mortier PH, Kiekens G, Auerbach RP, Cuijpers P, Demyttenaere K, Green JG, Nock MK, Kessler RC (2017) Stress impairs the efficacy of immune stimulation by CpG-C: potential neuroendocrine mediating mechanisms and significance to tumor metastasis and the perioperative period. Physiol Behav 176:139–148. https://doi.org/10.1016/j.bbi.2016.02.025.Stress
Haldar R et al (2020) Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer 126:3991–4001. https://doi.org/10.1002/cncr.32950
Drell TL IV et al (2003) Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat 80:63–70. https://doi.org/10.1023/A:1024491219366
Du P et al (2020) Chronic stress promotes EMT-mediated metastasis through activation of STAT3 signaling pathway by miR-337-3p in breast cancer. Cell Death Dis 11:1–13. https://doi.org/10.1038/s41419-020-02981-1
Shi M et al (2010) Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer 9:1–14. https://doi.org/10.1186/1476-4598-9-269
Yang EV et al (2006) Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 66:10357–10364. https://doi.org/10.1158/0008-5472.CAN-06-2496
Liu J et al (2020) A novel β2-AR/YB-1/β-catenin axis mediates chronic stress-associated metastasis in hepatocellular carcinoma. Oncogenesis. https://doi.org/10.1038/s41389-020-00268-w
Liu D et al (2020) β2-AR activation promotes cleavage and nuclear translocation of Her2 and metastatic potential of cancer cells. Cancer Sci 111:4417–4428. https://doi.org/10.1111/cas.14676
Lu Y et al (2022) Chronic stress model simulated by salbutamol promotes tumorigenesis of gastric cancer cells through β2-AR/ERK/EMT pathway. J Cancer 13:401–412. https://doi.org/10.7150/jca.65403
Zhang J et al (2016) Norepinephrine induced epithelial–mesenchymal transition in HT-29 and A549 cells in vitro. J Cancer Res Clin Oncol 142:423–435. https://doi.org/10.1007/s00432-015-2044-9
Lang K et al (2004) Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer 112:231–238. https://doi.org/10.1002/ijc.20410
Cheng Y et al (2018) Depression promotes prostate cancer invasion and metastasis via a sympathetic-cAMP-FAK signaling pathway. Oncogene 37:2953–2966. https://doi.org/10.1038/s41388-018-0177-4
Flint MS et al (2013) Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress 16:114–121. https://doi.org/10.3109/10253890.2012.686075
Nishida M, Kozakai T, Nagami K, Kanamaru Y, Yabe T (2014) Structural alteration of cell surface heparan sulfate through the stimulation of the signaling pathway for heparan sulfate 6-O-sulfotransferase-1 in mouse fibroblast cells. Biosci Biotechnol Biochem 78:770–779. https://doi.org/10.1080/09168451.2014.905178
Chen H et al (2018) Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol 244:49–60. https://doi.org/10.1002/path.4988
Hori Y et al (2011) Naftopidil, a selective α1-adrenoceptor antagonist, suppresses human prostate tumor growth by altering interactions between tumor cells and stroma. Cancer Prev Res 4:87–96. https://doi.org/10.1158/1940-6207.CAPR-10-0189
Hondermarck H, Jobling P (2018) The sympathetic nervous system drives tumor angiogenesis. Trends in Cancer 4:93–94. https://doi.org/10.1016/j.trecan.2017.11.008
Liu J et al (2015) The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 52:130–142. https://doi.org/10.1016/j.psyneuen.2014.11.008
Madden KS, Szpunar MJ, Brown EB (2011) β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res Treat 130:747–758. https://doi.org/10.1007/s10549-011-1348-y
Lu Y et al (2017) Isoprenaline/β2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesis. BMC Cancer 17:1–15. https://doi.org/10.1186/s12885-017-3894-0
Hulsurkar M et al (2017) Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene 36:1525–1536. https://doi.org/10.1038/onc.2016.319
Lutgendorf SK et al (2002) Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer 95:808–815. https://doi.org/10.1002/cncr.10739
Costanzo ES et al (2005) Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer 104:305–313. https://doi.org/10.1002/cncr.21147
Lutgendorf SK et al (2008) Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res 14:6839–6846. https://doi.org/10.1158/1078-0432.CCR-08-0230
Mulcrone PL et al (2017) Skeletal colonization by breast cancer cells is stimulated by an osteoblast and β2AR-dependent neo-angiogenic switch. J Bone Miner Res 32:1442–1454. https://doi.org/10.1002/jbmr.3133
Moreno-Smith M et al (2011) Dopamine blocks stress-mediated ovarian carcinoma growth. Clin Cancer Res 17:3649–3659. https://doi.org/10.1158/1078-0432.CCR-10-2441
Stacker SA et al (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14:159–172. https://doi.org/10.1038/nrc3677
Le CP et al (2016) Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. https://doi.org/10.1038/ncomms10634
Bower JE et al (2018) Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr 2:1–7. https://doi.org/10.1093/jncics/pky029
Corona-Pérez A et al (2017) Interactive effects of chronic stress and a high-sucrose diet on nonalcoholic fatty liver in young adult male rats. Stress 20:608–617. https://doi.org/10.1080/10253890.2017.1381840
Mir N, Chin SA, Riddell MC (2021) Genomic and non-genomic actions of glucocorticoids on adipose tissue lipid metabolism
Fan KQ et al (2019) Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179:864-879.e19. https://doi.org/10.1016/j.cell.2019.10.001
Saussez S et al (2014) Towards Neuroimmunotherapy for Cancer: the Neurotransmitters Glutamate, Dopamine and GnRH-II augment substantially the ability of T cells of few Head and Neck cancer patients to perform spontaneous migration, chemotactic migration and migration towards th. J Neural Transm 121:1007–1027. https://doi.org/10.1007/s00702-014-1242-y
Tang J, Li Z, Lu L, Cho CH (2013) β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol 23:533–542. https://doi.org/10.1016/j.semcancer.2013.08.009
Frick LR et al (2009) Involvement of thyroid hormones in the alterations of T-cell immunity and tumor progression induced by chronic stress. Biol Psychiatry 65:935–942. https://doi.org/10.1016/j.biopsych.2008.12.013
Arranz A et al (2010) The impact of stress on tumor growth: peripheral CRF mediates tumor-promoting effects of stress. Mol Cancer 9:1–13. https://doi.org/10.1186/1476-4598-9-261
Cao L et al (2010) Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142:52–64. https://doi.org/10.1016/j.cell.2010.05.029
Zuo X et al (2019) 5-Hydroxytryptamine receptor 1D aggravates hepatocellular carcinoma progression through FoxO6 in AKT-dependent and independent manners. Hepatology 69:2031–2047. https://doi.org/10.1002/hep.30430
Cheng Y et al (2019) Depression-induced neuropeptide y secretion promotes prostate cancer growth by recruiting myeloid cells. Clin Cancer Res 25:2621–2632. https://doi.org/10.1158/1078-0432.CCR-18-2912
Zhang S et al (2022) Neuroendocrine regulation of stress-induced T cell dysfunction during lung cancer immunosurveillance via the Kisspeptin/GPR54 signaling pathway. Adv Sci. https://doi.org/10.1002/advs.202104132
Bruffaerts R, Mortier PH, Kiekens G, Auerbach RP, Cuijpers P, Demyttenaere K, Green JG, Nock MK, Kessler RC (2017) Exploiting the critical perioperative period to improve long-term cancer outcomes. Physiol Behav 176:139–148. https://doi.org/10.1038/nrclinonc.2014.224.Exploiting
Cole SW (2013) Nervous system regulation of the cancer genome. Brain Behav Immun 30:1–20. https://doi.org/10.1016/j.bbi.2012.11.008
De Giorgi V et al (2018) Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol 4:2–5. https://doi.org/10.1001/jamaoncol.2017.2908
Schuller HM, Porter B, Riechert A (2000) Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol 126:624–630. https://doi.org/10.1007/PL00008474
Lamkin DM et al (2015) α2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology 51:262–270. https://doi.org/10.1016/j.psyneuen.2014.10.004
Wolter JK et al (2014) Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 5:161–172. https://doi.org/10.18632/oncotarget.1083
Shan T et al (2011) β2-Adrenoceptor blocker synergizes with gemcitabine to inhibit the proliferation of pancreatic cancer cells via apoptosis induction. Eur J Pharmacol 665:1–7. https://doi.org/10.1016/j.ejphar.2011.04.055
Zhao CM et al (2014) Denervation suppresses gastric tumorigenesis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3009569
Hara MR, Sachs BD, Caron MG, Lefkowitz RJ (2013) Pharmacological blockade of a β2AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 12:219–224. https://doi.org/10.4161/cc.23368
Botteri E et al (2013) Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat 140:567–575. https://doi.org/10.1007/s10549-013-2654-3
Knight JM et al (2020) Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv 4:467–476. https://doi.org/10.1182/bloodadvances.2019000765
Grytli HH, Fagerland MW, Taskén KA, Fosså SD, Håheim LL (2013) Use of β-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 73:250–260. https://doi.org/10.1002/pros.22564
de Giorgi V et al (2011) Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171:779–781. https://doi.org/10.1001/archinternmed.2011.131
Manuscript A (2015) Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. 40–47 https://doi.org/10.1016/j.bbi.2014.02.019.Chronic
Palm D et al (2006) The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. Int J Cancer 118:2744–2749. https://doi.org/10.1002/ijc.21723
Hollestein LM, De Vries E (2014) Effect of β-adrenergic blockers and other antihypertensive drugs on the risk of melanoma recurrence and death-II. Mayo Clin Proc 89:1165–1167. https://doi.org/10.1016/j.mayocp.2014.06.002
Melhem-Bertrandt A et al (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29:2645–2652. https://doi.org/10.1200/JCO.2010.33.4441
Powe DG et al (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1:628–638. https://doi.org/10.18632/oncotarget.197
Neeman E, Zmora O, Ben-Eliyahu S (2012) A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res 18:4895–4902. https://doi.org/10.1158/1078-0432.CCR-12-1087
Grytli HH, Fagerland MW, Fosså SD, Taskén KA (2014) Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 65:635–641. https://doi.org/10.1016/j.eururo.2013.01.007
Lipshultz SE, Cochran TR, Franco VI, Miller TL (2013) Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol 10:697–710. https://doi.org/10.1038/nrclinonc.2013.195
Pituskin E et al (2017) Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol 35:870–877. https://doi.org/10.1200/JCO.2016.68.7830
Guglin M et al (2019) Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 73:2859–2868. https://doi.org/10.1016/j.jacc.2019.03.495
Kokolus KM et al (2018) Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. https://doi.org/10.1080/2162402X.2017.1405205
Gary D. Friedman (2011) Norepinephrine antagonists and cancer risk. Bone 23:1–7. https://doi.org/10.1002/ijc.25351.Norepinephrine
Xu L et al (2014) COX-2 inhibition potentiates anti-angiogenic cancer therapy and prevents metastasis in preclinical models hhs public access one sentence summary: COX-2 blockade potentiates anti-angiogenic therapy. Sci Transl Med 6:242–284. https://doi.org/10.1126/scitranslmed.3008455.COX-2
Shaashua L et al (2017) Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res 23:4651–4661. https://doi.org/10.1158/1078-0432.CCR-17-0152
Antoni MH, Dhabhar FS (2019) The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 125:1417–1431. https://doi.org/10.1002/cncr.31943
Biondia M et al (1994) Personality, endocrine and immune changes after eight months in healthy individuals under normal daily stress. Psychother Psychosom 62:176–184. https://doi.org/10.1159/000288920
Antoni M et al (2012) Transcriptional modulation of human leukocytes by cognitive-behavioral stress management in women undergoing treatment for breast cancer. Biol Psychiatry 71:366–372. https://doi.org/10.1016/j.biopsych.2011.10.007.Cognitive-behavioral
Wootten AC et al (2015) Preliminary results of a randomised controlled trial of an online psychological intervention to reduce distress in men treated for localised prostate cancer. Eur Urol 68:471–479. https://doi.org/10.1016/j.eururo.2014.10.024
Witek Janusek L, Tell D, Mathews HL (2019) Mindfulness based stress reduction provides psychological benefit and restores immune function of women newly diagnosed with breast cancer: a randomized trial with active control. Brain Behav Immun 80:358–373. https://doi.org/10.1016/j.bbi.2019.04.012
Lengacher CA et al (2016) Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: a randomized controlled trial. J Clin Oncol 34:2827–2834. https://doi.org/10.1200/JCO.2015.65.7874
Chambers SK et al (2017) Mindfulness-based cognitive therapy in advanced prostate cancer: a randomized controlled trial. J Clin Oncol 35:291–297. https://doi.org/10.1200/JCO.2016.68.8788
Rodin G et al (2018) Managing Cancer and Living Meaningfully (CALM): A randomized controlled trial of a psychological intervention for patients with advanced cancer. J Clin Oncol 36:2422–2432. https://doi.org/10.1200/JCO.2017.77.1097
Breitbart W et al (2015) Meaning-centered group psychotherapy: An effective intervention for improving psychological well-being in patients with advanced cancer. J Clin Oncol 33:749–754. https://doi.org/10.1200/JCO.2014.57.2198
Carayol M et al (2013) Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: What is the optimal dose needed? Ann Oncol 24:291–300. https://doi.org/10.1093/annonc/mds342
Vodička M et al (2018) Microbiota affects the expression of genes involved in HPA axis regulation and local metabolism of glucocorticoids in chronic psychosocial stress. Brain Behav Immun 73:615–624. https://doi.org/10.1016/j.bbi.2018.07.007
Farzi A, Fröhlich EE, Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15:5–22. https://doi.org/10.1007/s13311-017-0600-5
O’Mahony SM et al (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65:263–267. https://doi.org/10.1016/j.biopsych.2008.06.026
Chevalier G et al (2020) Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. https://doi.org/10.1038/s41467-020-19931-2
Shan B et al (2020) Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway. Psychoneuroendocrinology 117:104699. https://doi.org/10.1016/j.psyneuen.2020.104699
Xu D et al (2014) Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 146:1–18. https://doi.org/10.1053/j.gastro.2013.10.026
Wong ML et al (2016) Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 21:797–805. https://doi.org/10.1038/mp.2016.46
Zhang Z et al (2020) Xiaoyaosan slows cancer progression and ameliorates gut dysbiosis in mice with chronic restraint stress and colorectal cancer xenografts. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2020.110916
Wang T et al (2021) Clostridium butyricum relieve the visceral hypersensitivity in mice induced by Citrobacter rodentium infection with chronic stress. PeerJ. https://doi.org/10.7717/peerj.11585
Burokas A et al (2017) Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 82:472–487. https://doi.org/10.1016/j.biopsych.2016.12.031
Peterson SC et al (2015) Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16:400–412. https://doi.org/10.1016/j.stem.2015.02.006
Jobling P et al (2015) Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res 75:1777–1781. https://doi.org/10.1158/0008-5472.CAN-14-3180
Bauman J, McVary K (2013) Autonomic nerve development contributes to prostate cancer progression. Asian J Androl 15:713–714. https://doi.org/10.1038/aja.2013.113
Monje M (2017) Settling a nervous stomach: the neural regulation of enteric cancer. Cancer Cell 31:1–2. https://doi.org/10.1016/j.ccell.2016.12.008
Renz BW et al (2018) β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33:75-90.e7. https://doi.org/10.1016/j.ccell.2017.11.007
Demont Y et al (2012) Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J Biol Chem 287:1923–1931. https://doi.org/10.1074/jbc.M110.211714
McCaffrey G et al (2014) NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res 74:7014–7023. https://doi.org/10.1158/0008-5472.CAN-14-1220
Logotheti S et al (2020) Neural networks recapitulation by cancer cells promotes disease progression: a novel role of p73 isoforms in cancer-neuronal crosstalk. Cancers (Basel) 12:1–12. https://doi.org/10.3390/cancers12123789
Zheng Z et al (2022) Hypothalamus-habenula potentiation encodes chronic stress experience and drives depression onset. Neuron. https://doi.org/10.1016/j.neuron.2022.01.011
Boilly B, Faulkner S, Jobling P, Hondermarck H (2017) Nerve dependence: from regeneration to cancer. Cancer Cell 31:342–354. https://doi.org/10.1016/j.ccell.2017.02.005
Obradović MMS et al (2019) Glucocorticoids promote breast cancer metastasis. Nature 567:540–544. https://doi.org/10.1038/s41586-019-1019-4
Brahmer JR et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36:1714–1768. https://doi.org/10.1200/JCO.2017.77.6385
Senthil Kumar KJ et al (2019) MicroRNA-708 activation by glucocorticoid receptor agonists regulate breast cancer tumorigenesis and metastasis via downregulation of NF-κB signaling. Carcinogenesis 40:335–348. https://doi.org/10.1093/carcin/bgz011
Flint MS, Baum A, Chambers WH, Jenkins FJ (2007) Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 32:470–479. https://doi.org/10.1016/j.psyneuen.2007.02.013
de La Roca-Chiapas JM et al (2016) Impact of stress and levels of corticosterone on the development of breast cancer in rats. Psychol Res Behav Manag 9:1–6. https://doi.org/10.2147/PRBM.S94177
Hassan SA et al (2021) Relationship between locomotor activity rhythm and corticosterone levels during HCC development, progression, and treatment in a mouse model. J Pineal Res 70:1–13. https://doi.org/10.1111/jpi.12724
Berry A et al (2021) Chronic isolation stress affects central neuroendocrine signaling leading to a metabolically active microenvironment in a mouse model of breast cancer. Front Behav Neurosci 15:1–14. https://doi.org/10.3389/fnbeh.2021.660738
Lim DW et al (2019) Administration of Asian herb bennet (Geum japonicum) extract reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Nutrients 11:1–12. https://doi.org/10.3390/nu11122841
Chudzik A, Orzyłowska A, Rola R, Stanisz GJ (2021) Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: modulation of the brain–gut–microbiome axis. Biomolecules. https://doi.org/10.3390/biom11071000
Article R (2008) Individual differences in pre-carcinogen cytokine and corticosterone concentrations and depressive-like behavior predict tumor onset in rats exposed to a carcinogen. Bone 23:1–7. https://doi.org/10.1016/j.psyneuen.2012.09.003.Individual
Yang H et al (2019) Stress–glucocorticoid–TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med 25:1428–1441. https://doi.org/10.1038/s41591-019-0566-4
Shpilberg Y, Connor MK, Riddell MC (2015) The direct and indirect effects of corticosterone and primary adipose tissue on MCF7 breast cancer cell cycle progression. Horm Mol Biol Clin Investig 22:91–100. https://doi.org/10.1515/hmbci-2015-0003
Words K et al (2019) Molecular mechanisms linking exercise to cancer prevention and treatment. Int J Mol Sci 20:1689–1699. https://doi.org/10.1017/CBO9781107415324.004
Mandelblatt JS et al (2011) Associations of physical activity with quality of life and functional ability in breast cancer patients during active adjuvant treatment: the Pathways Study. Breast Cancer Res Treat 129:521–529. https://doi.org/10.1007/s10549-011-1483-5
Moore SC et al (2016) Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 176:816–825. https://doi.org/10.1001/jamainternmed.2016.1548
Dethlefsen C et al (2016) Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat 159:469–479. https://doi.org/10.1007/s10549-016-3970-1
Nemet D et al (2002) Systemic vs. local cytokine and leukocyte responses to unilateral wrist flexion exercise. J Appl Physiol 93:546–554. https://doi.org/10.1152/japplphysiol.00035.2002
Idorn M, Hojman P (2016) Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med 22:565–577. https://doi.org/10.1016/j.molmed.2016.05.007
Rundqvist H et al (2020) Cytotoxic t-cells mediate exercise-induced reductions in tumor growth. Elife 9:1–25. https://doi.org/10.7554/eLife.59996
Liu C et al (2021) Environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat Commun 12:1–15. https://doi.org/10.1038/s41467-021-25967-9
Dethlefsen C et al (2017) Exercise-induced catecholamines activate the Hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res 77:4894–4904. https://doi.org/10.1158/0008-5472.CAN-16-3125
Friedenreich CM et al (2019) The effect of prescribed exercise volume on biomarkers of chronic stress in postmenopausal women: results from the Breast Cancer and Exercise Trial in Alberta (BETA). Prev Med Rep 15:100960. https://doi.org/10.1016/j.pmedr.2019.100960
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
This work was supported by the National Natural Science Foundation of China (No: 82073280 to WWH). Seeking Outstanding Youth Program, Lingang Laboratory (No: LG-QS-202205-09 to WWH). The National Key New Drug Innovation Program, the Ministry of Science and Technology of China (No: 2018ZX09201017-006 to YY). New Drug Leading Scholar Program, China Pharmaceutical University (to YY).
Author information
Authors and Affiliations
Contributions
CW,YMS and WWH contributed to writing this manuscript, CW and JPN contributed to searching for material. YY and WWH designed this manuscript. All authors listed have made a substantial contribution to the work. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent for publication
This manuscript has been approved for publication by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, C., Shen, Y., Ni, J. et al. Effect of chronic stress on tumorigenesis and development. Cell. Mol. Life Sci. 79, 485 (2022). https://doi.org/10.1007/s00018-022-04455-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04455-3