Abstract
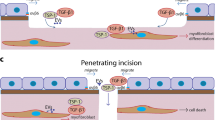
In the cornea, the epithelial basement membrane (EBM) and corneal endothelial Descemet’s basement membrane (DBM) critically regulate the localization, availability and, therefore, the functions of transforming growth factor (TGF)β1, TGFβ2, and platelet-derived growth factors (PDGF) that modulate myofibroblast development. Defective regeneration of the EBM, and notably diminished perlecan incorporation, occurs via several mechanisms and results in excessive and prolonged penetration of pro-fibrotic growth factors into the stroma. These growth factors drive mature myofibroblast development from both corneal fibroblasts and bone marrow-derived fibrocytes, and then the persistence of these myofibroblasts and the disordered collagens and other matrix materials they produce to generate stromal scarring fibrosis. Corneal stromal fibrosis often resolves completely if the inciting factor is removed and the BM regenerates. Similar defects in BM regeneration are likely associated with the development of fibrosis in other organs where perlecan has a critical role in the modulation of signaling by TGFβ1 and TGFβ2. Other BM components, such as collagen type IV and collagen type XIII, are also critical regulators of TGF beta (and other growth factors) in the cornea and other organs. After injury, BM components are dynamically secreted and assembled through the cooperation of neighboring cells—for example, the epithelial cells and keratocytes for the corneal EBM and corneal endothelial cells and keratocytes for the corneal DBM. One of the most critical functions of these reassembled BMs in all organs is to modulate the pro-fibrotic effects of TGFβs, PDGFs and other growth factors between tissues that comprise the organ.







Similar content being viewed by others
Availability of data and materials
NA.
Code availability
NA.
References
Sasaki T, Fässler R, Hohenester E (2004) Laminin: the crux of basement membrane assembly. J Cell Biol 164:959–963. https://doi.org/10.1083/jcb.200401058
Yurchenco PD, O’Rear J (1993) Supramolecular organization of basement membranes. In: Rohrbach DH, Timpl R (eds) Molecular and cellular aspects of basement membranes. Academic Press, San Diego, pp 20–47
Hohenester E, Yurchenco PD (2013) Laminins in basement membrane assembly. Cell Adh Migr 7:56–63. https://doi.org/10.4161/cam.21831
Wilson SE (2019) Coordinated modulation of corneal scarring by the epithelial basement membrane and Descemet’s basement membrane. J Refract Surg 35:506–516. https://doi.org/10.3928/1081597X-20190625-02
Martinez-Hernandez A, Amenta PS (1983) The basement membrane in pathology. Lab Invest 48:656–677
Miosge N (2001) The ultrastructural composition of basement membranes in vivo. Histol Histopathol 16:1239–1248. https://doi.org/10.14670/HH-16.1239
Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Bose K, Tam KP, Wilson SE (2017) Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res 161:101–105. https://doi.org/10.1016/j.exer.2017.05.003
Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE (2017) Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J Ref Surg 33:337–346. https://doi.org/10.3928/1081597X-20170126-02
Saikia P, Medeiros CS, Thangavadivel S, Wilson SE (2018) Basement membranes in the cornea and other organs that commonly develop fibrosis. Cell Tissue Res 374:439–453. https://doi.org/10.1007/s00441-018-2934-7
Schymeinsky J, Nedbal S, Miosge N, Pöschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL (2002) Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol 22:6820–6830. https://doi.org/10.1128/mcb.22.19.6820-6830.2002
Engel J (1993) Structure and function of laminin. In: Rohrbach DH (ed) Molecular and cellular aspects of basement membranes. Academic Press, San Diego, pp 147–176
Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD (2005) A simplified laminin nomenclature. Matrix Biol 24:326–332. https://doi.org/10.1016/j.matbio.2005.05.006
Cohen MW, Jacobson C, Yurchenco PD, Morris GE, Carbonetto S (1997) Laminin-induced clustering of dystroglycan on embryonic muscle cells: comparison with agrin-induced clustering. J Cell Biol 136:1047–1058. https://doi.org/10.1083/jcb.136.5.1047
Colognato H, Yurchenco PD (1999) The laminin α2 expressed by dystrophic dy2j mice is defective in its ability to form polymers. Curr Biol 9:1327–1330. https://doi.org/10.1016/s0960-9822(00)80056-1
Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE (1996) Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J Cell Biol 132:1189–1198. https://doi.org/10.1083/jcb.132.6.1189
Walko G, Castañón MJ, Wiche G (2015) Molecular architecture and function of the hemidesmosome. Cell Tissue Res 360:529–544. https://doi.org/10.1007/s00441-014-2061-z
Mayer U, Timpl R (1994) Nidogen, a versatile binding protein of basement membranes. In: Yurchenco PD, Birk DE, Mecham RP (eds) Extracellular Matrix Assembly and Structure. Academic Press, San Diego, pp 389–416
Kohfeld E, Sasaki T, Gohring W, Timpl R (1998) Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol 282:99–109. https://doi.org/10.1006/jmbi.1998.2004
Mann K, Deutzmann R, Aumailley M, Timpl R, Raimondi L, Yamada Y, Pan T-C, Conway D, Chu ML (1989) Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J 8:65–72
Murshed M, Smyth N, Miosge N, Karolat T, Krieg M, Paulsson M, Nischt R (2000) The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol 20:7007–7012. https://doi.org/10.1128/mcb.20.18.7007-7012.2000
Shibuya H, Okamoto O, Fujiwara S (2006) The bioactivity of transforming growth factor-beta1 can be regulated via binding to dermal collagens in mink lung epithelial cells. J Dermatol Sci 41:187–195. https://doi.org/10.1016/j.jdermsci.2005.10.005
Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R (2005) Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol 25:6846–6856. https://doi.org/10.1128/MCB.25.15.6846-6856.2005
Timpl R (1994) Proteoglycans of basement membranes. EXS 70:123–144. https://doi.org/10.1007/978-3-0348-7545-5_8
Iozzo RV (2005) Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 6:646–656. https://doi.org/10.1038/nrm1702
Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assmann KJ, Monnens LA, Veerkamp JH, van den Heuvel LP (1998) Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem 46:19–27. https://doi.org/10.1177/002215549804600104
Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R (1999) Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 147:1109–1122. https://doi.org/10.1083/jcb.147.5.1109
Hassel JR, Gehron Robey P, Barrach HJ, Wilczeck J, Rennard SI, Martin GR (1980) Isolation of heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci USA 77:4494–4498. https://doi.org/10.1073/pnas.77.8.4494
Noonan D, Hassel JR (1993) Proteoglycans of basement membranes. In: Rohrback DH (ed) Molecular and cellular aspects of basement membranes. Academic Press, San Diego, pp 189–210
Denzer AJ, Gesemann M, Schumacher B, Ruegg MA (1995) An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J Cell Biol 131:1547–1560. https://doi.org/10.1083/jcb.131.6.1547
Hudson BG, Reeders ST, Tryggvason K (1993) Type IV collagen: structure, gene organization and role in human diseases. J Biol Chem 268:26033–26036
Khoshnoodi J, Pedchenko V, Hudson BG (2008) Mammalian collagen IV. Microsc Res Tech 71:357–370. https://doi.org/10.1002/jemt.20564
Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC (1995) Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 72:461–473
Quondamatteo F (2002) Assembly, stability and integrity of basement membrane in vivo. Histochem J 34:369–381. https://doi.org/10.1023/a:1023675619251
Gatseva A, Sin YY, Brezzo G, Van Agtmael T (2019) Basement membrane collagens and disease mechanisms. Essays Biochem 63:297–312. https://doi.org/10.1042/EBC20180071
Calvo AC, Moreno L, Moreno L, Toivonen JM, Manzano R, Molina N, de la Torre M, López T, Miana-Mena FJ, Muñoz MJ, Zaragoza P, Larrodé P, García-Redondo A, Osta R (2020) Type XIX collagen: a promising biomarker from the basement membranes. Neural Regen Res 15:988–995. https://doi.org/10.4103/1673-5374.270299
Kato T, Chang JH, Azar DT (2003) Expression of type XVIII collagen during healing of corneal incisions and keratectomy wounds. Invest Ophthalmol Vis Sci 44:78–85. https://doi.org/10.1167/iovs.01-1257
Cotrufo M, De Santo L, Della Corte A, Di Meglio F, Guerra G, Quarto C, Vitale S, Castaldo C, Montagnani S (2005) Basal lamina structural alterations in human asymmetric aneurismatic aorta. Eur J Histochem 49:363–370. https://doi.org/10.4081/964
Löffek S, Schilling O, Franzke CW (2011) Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38:191–208. https://doi.org/10.1183/09031936.00146510
de Oliveira RC, Sampaio LP, Shiju TM, Santhiago MR, Wilson SE (2022) Epithelial basement membrane regeneration after PRK-induced epithelial-stromal injury in rabbits: fibrotic vs. non-fibrotic corneal healing. J Ref Surg 38:50–60
Wilson SE (2021) TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp Eye Res 207:108594. https://doi.org/10.1016/j.exer.2021.108594
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839. https://doi.org/10.1161/01.RES.0000070112.80711.3D
Nakayama K (1997) Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J 327(Pt 3):625–635. https://doi.org/10.1042/bj3270625
Caley MP, Martins VL, O’Toole EA (2015) Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 4:225–234. https://doi.org/10.1089/wound.2014.0581
Sivak JM, Fini ME (2002) MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res 21:1–14. https://doi.org/10.1016/s1350-9462(01)00015-5
Geanon JD, Tripathi BJ, Tripathi RC, Barlow GH (1987) Tissue plasminogen activator in avascular tissues of the eye: a quantitative study of its activity in the cornea, lens, and aqueous and vitreous humors of dog, calf, and monkey. Exp Eye Res 44:55–63. https://doi.org/10.1016/s0014-4835(87)80025-8
Sugioka K, Mishima H, Kodama A, Itahashi M, Fukuda M, Shimomura Y (2016) Regulatory mechanism of collagen degradation by keratocytes and corneal inflammation: the role of urokinase-type plasminogen activator. Cornea 35(Suppl 1):S59–S64. https://doi.org/10.1097/ICO.0000000000000995
Tervo T, Tervo K, van Setten GB, Virtanen I, Tarkkanen A (1989) Plasminogen activator and its inhibitor in the experimental corneal wound. Exp Eye Res 48:445–449. https://doi.org/10.1016/s0014-4835(89)80012-0
de Oliveira RC, Tye G, Sampaio LP, Shiju TM, Dedreu J, Menko AS, Santhiago MR, Wilson SE (2021) TGFβ1 and TGFβ2 proteins in corneas with and without stromal fibrosis: delayed regeneration of epithelial barrier function and the epithelial basement membrane in corneas with stromal fibrosis. Exp Eye Res 202:108325. https://doi.org/10.1016/j.exer.2020.108325
Schittny JC, Yurchenco PD (1989) Basement membranes: molecular organization and function in development and disease. Curr Opin Cell Biol 1:983–988. https://doi.org/10.1016/0955-0674(89)90069-0
Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD (2003) The role of laminin in embryonic cell polarization and tissue organization. Dev Cell 4:613–624. https://doi.org/10.1016/s1534-5807(03)00128-x
Miner JH, Li C, Mudd JL, Go G, Sutherland AE (2004) Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131:2247–2256. https://doi.org/10.1242/dev.01112
Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D (1999) Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 144:151–160. https://doi.org/10.1083/jcb.144.1.151
Yurchenco PD, Furthmayr H (1984) Self-assembly of basement membrane collagen. Biochemistry 23:1839–1850. https://doi.org/10.1021/bi00303a040
Grant DS, Leblond CP, Kleinmann HK, Inoue S, Hassell J (1989) The incubation of laminin, collagen IV and heparan sulfate proteoglycan at 35◦C yields basement membrane-like structures. J Cell Biol 108:1567–1574. https://doi.org/10.1083/jcb.108.4.1567
Yurchenco PD, Ruben GC (1987) Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol 105:2559–2568. https://doi.org/10.1083/jcb.105.6.2559
Yurchenco PD, Schittny JC (1990) Molecular architecture of basement membranes. FASEB J 4:1577–1590. https://doi.org/10.1096/fasebj.4.6.2180767
Yurchenco PD, O’Rear JJ (1994) Basal lamina assembly. Curr Opin Cell Biol 6:674–681. https://doi.org/10.1016/0955-0674(94)90093-0
Paulsson M (1988) The role of Ca2+ binding in the self-aggregation of laminin-nidogen complexes. J Biol Chem 263:5425–5430
Yurchenco PD (2015) Integrating activities of laminins that drive basement membrane assembly and function. Curr Top Membr 76:1–30. https://doi.org/10.1016/bs.ctm.2015.05.001
Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H (1985) Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem 260:7636–7644
Yurchenco PD, Cheng YS, Colognato H (1992) Laminin forms an independent network in basement membranes. J Cell Biol 117:1119–1133. https://doi.org/10.1083/jcb.117.5.1119
Dziadek M (1995) Role of laminin-nidogen complexes in basementmembrane formation during embryonic development. Experientia 51:901–913. https://doi.org/10.1007/BF01921740
Timpl R, Brown JC (1996) Supramolecular assembly of basement membranes. BioEssays 18:123–32. https://doi.org/10.1002/bies.950180208
Marnkovich MP, Keene DR, Rimberg CS, Burgeson RE (1993) Cellular origin of the dermal-epidermal basement membrane. Dev Dyn 197:255–267. https://doi.org/10.1002/aja.1001970404
Breitkreutz D, Mirancea M, Schmidt C, Beck R, Werner U, Stark H-J, Gerl M, Fusenig NE (2004) Inhibition of basement membrane formation by a nidogen-binding laminin gamma1-chain fragment in human skin-organotypic co-cultures. J Cell Sci 117:2611–2622. https://doi.org/10.1242/jcs.01127
Simon-Assmann P, Bouziges F, Arnold C, Haffen K, Kedinger M (1988) Epithelial-mesenchymal interactions in the production of basement membrane components in the gut. Development 102:339–347
Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE (2015) Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp Eye Res 134:33–38. https://doi.org/10.1016/j.exer.2015.03.016
Wilson SE, Medeiros CS, Santhiago MR (2018) Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries. J Ref Surg 34:59–64. https://doi.org/10.3928/1081597X-20171128-01
Shiju TM, de Oliveira RC, Wilson SE (2020) 3D in vitro corneal models: a review of current technologies. Exp Eye Res 200:108213. https://doi.org/10.1016/j.exer.2020.108213
Santhanam A, Marino GK, Torricelli AAM, Wilson SE (2017) EBM regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol Vis 23:39–51
Santhanam A, Torricelli AAM, Wu J, Marino GK, Wilson SE (2015) Differential expression of epithelial basement membrane components nidogens and perlecan in corneal stromal cells in vitro. Mol Vis 21:1318–1327
Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE (2013) Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophth Vis Sci 54:4026–4033. https://doi.org/10.1167/iovs.13-12106
Torricelli AAM, Singh V, Santhiago MR, Wilson SE (2013) The corneal epithelial basement membrane: structure, function and disease. Invest Ophth Vis Sci 54:6390–6400. https://doi.org/10.1167/iovs.13-12547
Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE (2018) Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp Eye Res 170:177–187. https://doi.org/10.1016/j.exer.2018.02.018
Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, Siebuhr A, Gudmann NS, Rønnow S, Sand JM, Daniels SJ, Mortensen JH, Schuppan D (2017) The good and the bad collagens of fibrosis—their role in signaling and organ function. Adv Drug Deliv Rev 121:43–56. https://doi.org/10.1016/j.addr.2017.07.014
Saikia P, Crabb JS, Dibbin LL, Juszczak MJ, Willard B, Jang GF, Shiju TM, Crabb JW, Wilson SE (2020) Quantitative proteomic comparison of myofibroblasts derived from bone marrow and cornea. Sci Rep 10:16717. https://doi.org/10.1038/s41598-020-73686-w
Saikia P, Thangavadivel S, Lassance L, Medeiros CS, Wilson SE (2018) IL-1 and TGF-β modulation of epithelial basement membrane components perlecan and nidogen production by corneal stromal cells. Invest Ophth Vis Sci 59:5589–5598. https://doi.org/10.1167/iovs.18-25202
Sampaio LP, Shiju TM, Hilgert GSL, de Oliveira RC, DeDreu J, Menko AS, Santhiago MR, Wilson SE (2021) Descemet’s membrane injury and regeneration, and posterior corneal fibrosis, in rabbits. Exp Eye Res 213:108803. https://doi.org/10.1016/j.exer.2021.108803
Kisling A, Lust RM, Katwa LC (2019) What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci 228:30–34. https://doi.org/10.1016/j.lfs.2019.04.042
Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE (2006) Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res 82:788–797. https://doi.org/10.1016/j.exer.2005.09.021
Wilson SE, He Y-G, Weng J, Li Q, McDowall AW, Vital M, Chwang EL (1996) Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res 62:325–328. https://doi.org/10.1006/exer.1996.0038
Mohan RR, Hutcheon AEK, Choi R, Hong J-W, Lee J-S, Mohan RR, Ambrósio R, Zieske JD, Wilson SE (2003) Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res 76:71–87. https://doi.org/10.1016/s0014-4835(02)00251-8
Wilson SE, Marino GK, Torricelli AAM, Medeiros CS (2017) Corneal fibrosis: injury and defective regeneration of the epithelial basement membrane. A paradigm for fibrosis in other organs? Matrix Biol 64:17–26. https://doi.org/10.1016/j.matbio.2017.06.003
Medeiros CS, Saikia P, de Oliveira RC, Lassance L, Santhiago MR, Wilson SE (2019) Descemet’s membrane modulation of posterior corneal fibrosis. Invest Ophth Vis Sci 60:1010–1020. https://doi.org/10.1167/iovs.18-26451
Ramos-Lewis W, LaFever KS, Page-McCaw A (2018) A scar-like lesion is apparent in basement membrane after wound repair in vivo. Matrix Biol 74:101–120. https://doi.org/10.1016/j.matbio.2018.07.004
Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K (2006) Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol 25:189–197. https://doi.org/10.1016/j.matbio.2005.12.001
Deutzmann R, Aumailley M, Wiedemann H, Pysny W, Timpl R, Edgar D (1990) Cell adhesion, spreading and neurite stimulation by laminin fragment E8 depends on maintenance of secondary and tertiary structure in its rod and globular domain. Eur J Biochem 191:513–522. https://doi.org/10.1111/j.1432-1033.1990.tb19151.x?sid=nlm%3Apubmed
Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, Li S, Wada Y, Combs AC, Ervasti JM, Sekiguchi K (2004) Molecular dissection of the a-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem 279:10946–10954. https://doi.org/10.1074/jbc.M313626200
Ido H, Nakamura A, Kobayashi R, Ito S, Li S, Futaki S, Sekiguchi K (2007) The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin c chains in integrin binding by laminins. J Biol Chem 282:11144–11154. https://doi.org/10.1074/jbc.M609402200
Ott U, Odermatt E, Engel J, Furthmayr H, Timpl R (1982) Protease resistance and conformation of laminin. Eur J Biochem 123:63–72. https://doi.org/10.1111/j.1432-1033.1982.tb06499.x
Smirnov SP, McDearmon EL, Li S, Ervasti JM, Tryggvason K, Yurchenco PD (2002) Contributions of the LG modules and furin processing to laminin-2 functions. J Biol Chem 277:18928–18937. https://doi.org/10.1074/jbc.M201880200
Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S (1993) Laminin-binding protein 120 from brain is closely related to the dystrophin associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem 268:14972–14980
Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R (1996) Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122:3537–3547
DiPersio CM, Hodivala-Dilke K, Jaenisch R, Kreidberg JA, Hynes RO (1997) α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 137:729–742. https://doi.org/10.1083/jcb.137.3.729
Sasaki T, Forsberg E, Bloch W, Addicks K, Fassler R, Timpl R (1998) Deficiency of β1 integrins in teratoma interferes with basement membrane assembly and laminin-1 expression. Exp Cell Res 238:70–81. https://doi.org/10.1006/excr.1997.3837
Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fässler R (2000) Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J 19:3990–4003. https://doi.org/10.1093/emboj/19.15.3990
Vidal F, Aberdam D, Miquel C, Christiano AM, Pulkkinen L, Uitto J, Ortonne P, Meneguzzi G (1995) Integrin β4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genetics 10:229–234. https://doi.org/10.1038/ng0695-229
Dowling J, Yu QC, Fuchs E (1996) β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol 134:559–572. https://doi.org/10.1083/jcb.134.2.559
Timpl R (1993) Proteoglycans of basement membranes. Experientia 49:417–427. https://doi.org/10.1007/BF01923586
Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP (1997) Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Gen 6:831–841. https://doi.org/10.1093/hmg/6.6.831
Henry MD, Campbell KP (1998) A role of dystroglycan in basement membrane assembly. Cell 95:859–870. https://doi.org/10.1016/s0092-8674(00)81708-0
Henry MD, Satz J, Brakebusch C, Costell M, Gustafsson E, Fassler R, Campbell KP (2001) Distinct roles for dystroglycan, β1-integrin and perlecan in cell surface laminin organization. J Cell Sci 114:1137–1144
Montanaro F, Lindenbaum M, Carbonetto S (1999) α-dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J Cell Biol 145:1325–1340. https://doi.org/10.1083/jcb.145.6.1325
Kalb E, Engel J (1991) Binding and calcium-induced aggregation of laminin onto lipid bilayers. J Biol Chem 266:19047–19052
Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K, Uitto J (1994) Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet 6:293–298. https://doi.org/10.1038/ng0394-293
Pulkkinen L, Christiano AM, Gerecke DR, Wagman DW, Burgeson RE, Pittelkow MR, Uitto J (1994) A homozygous nonsense mutation in the beta 3 gene of laminin 5 (LAMB3) in Herlitz junctional epidermolysis bullosa. Genomics 24:257–360. https://doi.org/10.1006/geno.1994.1627
Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131:1619–1628. https://doi.org/10.1242/dev.01037
Bezakova G, Rüegg MA (2003) New insights into the roles of agrin. Nat Rev Mol Cell Biol 4:295–308. https://doi.org/10.1038/nrm1074
Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J (1991) Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J 10:3137–3146. https://doi.org/10.1002/j.1460-2075.1991.tb04875.x
Hopf M, Göhring W, Ries A, Timpl R, Hohenester, (2001) Crystal structure and mutational analysis of a perlecan binding fragment of nidogen-1. Nat Struct Biol 8:634–640. https://doi.org/10.1038/89683
Mayer U, Nischt R, Pöschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R (1993) A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J 12:1879–1885. https://doi.org/10.1002/j.1460-2075.1993.tb05836.x
Pozzi A, Yurchenco PD, Iozzo RV (2017) The nature and biology of basement membranes. Matrix Biol 57–58:1–11. https://doi.org/10.1016/j.matbio.2016.12.009
Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, Bruckner-Tuderman L, Bruckner P, Hansen U (2012) The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J Biol Chem 287:18700–18709. https://doi.org/10.1074/jbc.M111.336073
Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357. https://doi.org/10.1016/s0092-8674(00)81279-9
Paralkar VM, Vukicevic S, Reddi AH (1991) Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol 143:303–308. https://doi.org/10.1016/0012-1606(91)90081-d
Iozzo RV, Zoeller JJ, Nystrom A (2009) Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol Cells 27:503–513. https://doi.org/10.1007/s10059-009-0069-0
Gohring W, Sasaki T, Heldin CH, Timpl R (1998) Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur J Biochem. 255:60–66. https://doi.org/10.1046/j.1432-1327.1998.2550060.x?sid=nlm%3Apubmed
Wilson SE (2021) Interleukin-1 and transforming growth factor beta: Commonly opposing, but sometimes supporting, master regulators of the corneal wound healing response to injury. Invest Ophth Vis Sci 62:8. https://doi.org/10.1167/iovs.62.4.8
Ruoslahti E, Yamaguchi Y (1991) Proteoglycans as modulators of growth factor activities. Cell 64:867–869. https://doi.org/10.1016/0092-8674(91)90308-l
Schlessinger J, Lax I, Lemmon M (1995) Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell 83:357–360. https://doi.org/10.1016/0092-8674(95)90112-4
Wilson SE, Walker JW, Chwang EL, He Y-G (1993) Hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), their receptors, FGF receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci 34:2544–2561
Wilson SE, He Y-G, Weng J, Zeiske JD, Jester JV, Schultz GS (1994) Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility, and differentiation of human corneal epithelial cells. Exp Eye Res 59:665–678. https://doi.org/10.1006/exer.1994.1152
Liang Q, Mohan RR, Chen L, Wilson SE (1998) Signaling by HGF and KGF in corneal epithelial cells: Ras/MAP kinase and Jak-STAT pathways. Invest Ophthalmol Vis Sci 39:1329–1338
Kakazu A, Chandrasekher G, Bazan HE (2004) HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad- but not the ERK1/2-mediated signaling pathway. Invest Ophthalmol Vis Sci 45:3485–3492. https://doi.org/10.1167/iovs.04-0372
Chandrasekher G, Pothula S, Maharaj G, Bazan HE (2014) Differential effects of hepatocyte growth factor and keratinocyte growth factor on corneal epithelial cell cycle protein expression, cell survival, and growth. Mol Vis 20:24–37
Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J (1999) The cellular basis of corneal transparency: evidence for “corneal crystallins.” J Cell Sci 112:613–622
Funding
VR180066 (SEW) from the Department of Defense, EY025585 from the National Eye Institute, and Research to Prevent Blindness, New York, NY.
Author information
Authors and Affiliations
Contributions
SEW conceived of and wrote this review article.
Corresponding author
Ethics declarations
Conflict of interest
The author does not have any commercial or proprietary interests in the subject matter of this review article.
Ethics approval
All animal experiments described in this review had Cleveland Clinic Institutional Animal Care and Use Committee and/or U.S Army Animal Care and Use Committee approval. All human studies had Investigational Review Board approval.
Consent to participate
NA.
Consent for publication
NA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wilson, S.E. Defective perlecan-associated basement membrane regeneration and altered modulation of transforming growth factor beta in corneal fibrosis. Cell. Mol. Life Sci. 79, 144 (2022). https://doi.org/10.1007/s00018-022-04184-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04184-7