Abstract
Breast cancer is the leading cause of cancer death in female. Until now, advanced breast cancer is still lack effective treatment strategies and reliable prognostic markers. In the present article, we introduced the physiologic and pathologic functions and regulation mechanisms of ZBTB28, a tumor suppressor gene, in breast cancer. ZBTB28 is frequently silenced in breast cancer due to promoter CpG methylation, and its expression is positively correlated with breast cancer patient survival. The antineoplastic effect of ZBTB28 in breast cancer was elucidated through a series of in vitro and in vivo measurements, including cell proliferation, apoptosis, cell cycle, epithelial mesenchymal transition (EMT), and growth of xenografts. Furthermore, ZBTB28 can directly regulate IFNAR to activate interferon-stimulated genes and potentiate macrophage activation. Ectopic ZBTB28 expression in breast cancer cells was sufficient to downregulate CD24 and CD47 to promote phagocytosis of macrophages, demonstrating that ZBTB28 was beneficial for the combination treatment of anti-CD24 and anti-CD47. Collectively, our results reveal a mode of action of ZBTB28 as a tumor suppressor gene and suggest that ZBTB28 is an important regulator of macrophage phagocytosis in breast cancer, holding promise for the development of novel therapy strategies for breast cancer patients.

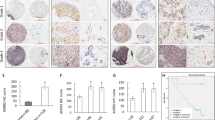
available at UALCAN databases showing ZBTB28 expression and methylation in Luminal, HER2 positive and TNBC types of breast cancer tissues compared to normal mammary tissues. D The relationship between ZBTB28 expression and survival of patients with breast cancer were illustrated through Kaplan–Meier plots database. E The expression and CpG methylation level of ZBTB28 in normal breast cell line and several kinds of breast cancer cell lines. β-actin expression as control. F Western blot analysis confirmed the exogenous expression of ZBTB28 in normal breast cells and breast cancer cell lines, with GAPDH as a control. G ZBTB28 methylation in primary breast cancer tissues (n = 34) and normal breast tissues (n = 16) were measured by MSP (M methylated; U unmethylated)






Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 5-Aza:
-
5-Aza-2’-deoxycytidine
- BrCa:
-
Breast cancer
- ChIP:
-
Chromatin immunoprecipitation
- EMT:
-
Epithelial–mesenchymal transition
- IFNAR:
-
The type I IFN receptor
- ISGs:
-
Interferon-stimulated genes
- MSP:
-
Methylation-specific PCR
- PMA:
-
12-Myristate 13-acetate
- Siglec-10:
-
Sialic-acid-binding Ig-like lectin 10
- SIRPα:
-
Signal regulatory protein alpha
- TFBSs:
-
Transcription factor-binding sites
- ZBTB28:
-
Zinc-finger and BTB/POZ (Poxvirus and Zinc-finger) domain-containing family protein 28
References
Weigelt B, Reis-Filho JS (2009) Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol 6(12):718–730. https://doi.org/10.1038/nrclinonc.2009.166
Bilimoria MM, Morrow M (1995) The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin 45(5):263–278
Tichy JR, Lim E, Anders CK (2013) Breast cancer in adolescents and young adults: a review with a focus on biology. J Nat Compr Cancer Netw JNCCN 11(9):1060–1069
Martin TA, Goyal A, Watkins G, Jiang WG (2005) Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol 12(6):488–496. https://doi.org/10.1245/aso.2005.04.010
Martin TD, Patel RS, Cook DR, Choi MY, Patil A, Liang AC et al (2021) The adaptive immune system is a major driver of selection for tumor suppressor gene inactivation. Science 373(6561):1327–1335. https://doi.org/10.1126/science.abg5784
Hartatik T, Okada S, Okabe S, Arima M, Hatano M, Tokuhisa T (2001) Binding of BAZF and Bc16 to STAT6-binding DNA sequences. Biochem Biophys Res Commun 284(1):26–32
Sakashita C, Fukuda T, Okabe S, Kobayashi H, Hirosawa S, Tokuhisa T, Miyasaka N, Miura O, Miki T (2002) Cloning and characterization of the human BAZF gene, a homologue of the BCL6 oncogene. Biochem Biophys Res Commun 291(3):567–573
Wang W, Huang P, Panyisha W, Kong R, Jiang X, Zhang L, Yang Q, Xie Q et al (2015) BCL6B expression in hepatocellular carcinoma and its efficacy in the inhibition of liver damage and fibrogenesis. Oncotarget 6(24):20252–20265
Hu S, Cao B, Zhang M, Linghu E, Zhan Q et al (2015) Epigenetic silencing BCL6B induced colorectal cancer proliferation and metastasis by inhibiting P53 signaling. Am J Cancer Res 5(2):651–662
Li L, Gong Y, Xu K, Chen W, Xia J, Cheng Z et al (2021) ZBTB28 induces autophagy by regulation of FIP200 and Bcl-XL facilitating cervical cancer cell apoptosis. J Exp Clin Cancer Res 40(1):150. https://doi.org/10.1186/s13046-021-01948-0
Yu LY, Tang J, Zhang CM, Zeng WJ, Yan H, Li MP et al (2017) new immunotherapy strategies in breast cancer. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph14010068
Berger A, Colpitts SJ, Seabrook MSS, Furlonger CL, Bendix MB, Moreau JM et al (2019) Interleukin-15 in cancer immunotherapy: IL-15 receptor complex versus soluble IL-15 in a cancer cell-delivered murine leukemia model. J Immunother Cancer 7(1):355. https://doi.org/10.1186/s40425-019-0777-8
Tang H, Qiao J, Fu YX (2016) Immunotherapy and tumor microenvironment. Cancer Lett 370(1):85–90. https://doi.org/10.1016/j.canlet.2015.10.009
Salvagno C, Ciampricotti M, Tuit S, Hau CS, van Weverwijk A, Coffelt SB et al (2019) Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol 21(4):511–521. https://doi.org/10.1038/s41556-019-0298-1
Mesev EV, LeDesma RA, Ploss A (2019) Decoding type I and III interferon signalling during viral infection. Nat Microbiol 4(6):914–924. https://doi.org/10.1038/s41564-019-0421-x
Xuan C, Steward KK, Timmerman JM, Morrison SL (2010) Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood 115(14):2864–2871. https://doi.org/10.1182/blood-2009-10-250555
Pirruccello SJ, LeBien TW (1986) The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol 136(10):3779–3784
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW et al (2019) CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572(7769):392–396. https://doi.org/10.1038/s41586-019-1456-0
Smith SC, Oxford G, Wu Z, Nitz MD, Conaway M, Frierson HF et al (2006) The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res 66(4):1917–1922. https://doi.org/10.1158/0008-5472.Can-05-3855
Kristiansen G, Sammar M, Altevogt P (2004) Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol 35(3):255–262. https://doi.org/10.1023/b:hijo.0000032357.16261.c5
Wang L, Liu R, Ye P, Wong C, Chen GY, Zhou P et al (2015) Intracellular CD24 disrupts the ARF-NPM interaction and enables mutational and viral oncogene-mediated p53 inactivation. Nat Commun 6:5909. https://doi.org/10.1038/ncomms6909
Brown EJ, Frazier WA (2001) Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11(3):130–135. https://doi.org/10.1016/s0962-8924(00)01906-1
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS et al (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA 109(17):6662–6667. https://doi.org/10.1073/pnas.1121623109
Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R et al (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138(2):271–285. https://doi.org/10.1016/j.cell.2009.05.046
Feng R, Zhao H, Xu J, Shen C (2020) CD47: the next checkpoint target for cancer immunotherapy. Crit Rev Oncol Hematol 152:103014. https://doi.org/10.1016/j.critrevonc.2020.103014
Qu M, Zou X, Fang F, Wang S, Xu L, Zeng Q et al (2020) Platelet-derived microparticles enhance megakaryocyte differentiation and platelet generation via miR-1915-3p. Nat Commun 11(1):4964. https://doi.org/10.1038/s41467-020-18802-0
Xiang T, Tang J, Li L, Peng W, Du Z, Wang X et al (2019) Tumor suppressive BTB/POZ zinc-finger protein ZBTB28 inhibits oncogenic BCL6/ZBTB27 signaling to maintain p53 transcription in multiple carcinogenesis. Theranostics 9(26):8182–8195. https://doi.org/10.7150/thno.34983
Zhang Y, Fan J, Fan Y, Li L, He X, Xiang Q et al (2018) The new 6q27 tumor suppressor DACT2, frequently silenced by CpG methylation, sensitizes nasopharyngeal cancer cells to paclitaxel and 5-FU toxicity via beta-catenin/Cdc25c signaling and G2/M arrest. Clin Epigenetics 10(1):26. https://doi.org/10.1186/s13148-018-0459-2
Li L, Tao Q, Jin H, van Hasselt A, Poon FF, Wang X et al (2010) The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res 16(11):2949–2958. https://doi.org/10.1158/1078-0432.ccr-09-3178
Lu W, Kang Y (2019) Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell 49(3):361–374. https://doi.org/10.1016/j.devcel.2019.04.010
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5):576–590. https://doi.org/10.1016/j.ccr.2011.09.009
Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL et al (2019) Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 68(9):1653–1666. https://doi.org/10.1136/gutjnl-2019-318419
Casazza A, Mazzone M (2014) Altering the intratumoral localization of macrophages to inhibit cancer progression. Oncoimmunology 3(1):e27872. https://doi.org/10.4161/onci.27872
Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B et al (1995) BCL-6 protein is expressed in germinal-center B cells. Blood 86(1):45–53
Herdoiza Padilla E, Crauwels P, Bergner T, Wiederspohn N, Förstner S, Rinas R et al (2019) mir-124-5p regulates phagocytosis of human macrophages by targeting the actin cytoskeleton via the ARP2/3 complex. Front Immunol 10:2210. https://doi.org/10.3389/fimmu.2019.02210
Borden EC (2019) Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov 18(3):219–234. https://doi.org/10.1038/s41573-018-0011-2
Parker BS, Rautela J, Hertzog PJ (2016) Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer 16(3):131–144. https://doi.org/10.1038/nrc.2016.14
Aricò E, Castiello L, Capone I, Gabriele L, Belardelli F (2019) Type I interferons and cancer: an evolving story demanding novel clinical applications. Cancers (Basel). https://doi.org/10.3390/cancers11121943
Kulkarni A, Chandrasekar V, Natarajan SK, Ramesh A, Pandey P, Nirgud J et al (2018) A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng 2(8):589–599. https://doi.org/10.1038/s41551-018-0254-6
Murata Y, Saito Y, Kotani T, Matozaki T (2018) CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci 109(8):2349–2357. https://doi.org/10.1111/cas.13663
Acknowledgements
This study was supported by National Natural Science Foundation of China (#81872380, #81572769, #82003135), Natural Science Foundation of Chongqing (2020ZYO13799), and Hong Kong-RGC (GRF# 14115019). The authors thank Prof. Qian Tao (the Chinese University of Hong Kong, Hong Kong, China) for generously providing primers and plasmids.
Author information
Authors and Affiliations
Contributions
LL and XT: conception and design. LL, GY, and TJ performed majority of experiments. PW, CZ, YR, DC, and LL performed experiments and analyzed data. XQ, MJ, YC, XJ, and WY collected samples. LL and GY drafted the manuscript. XT, LL, and GY reviewed data and manuscript. XT, LL, GY, and LX reviewed data and finalized the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Ethical approval and consent to participate
The study was authorized by the Institutional Ethics Committees of The First Affiliated Hospital of Chongqing Medical University and conformed to the principles of the Declaration of Helsinki.
Consent for publication
We have obtained consent to publish from the participant to report individual patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, L., Gong, Y., Tang, J. et al. ZBTB28 inhibits breast cancer by activating IFNAR and dual blocking CD24 and CD47 to enhance macrophages phagocytosis. Cell. Mol. Life Sci. 79, 83 (2022). https://doi.org/10.1007/s00018-021-04124-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-021-04124-x