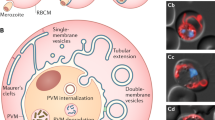
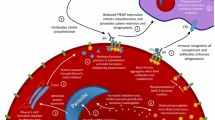
Abstract
Malaria is a vector-borne parasitic disease with a vast impact on human history, and according to the World Health Organisation, Plasmodium parasites still infect over 200 million people per year. Plasmodium falciparum, the deadliest parasite species, has a remarkable ability to undermine the host immune system and cause life-threatening disease during blood infection. The parasite’s host cells, red blood cells (RBCs), generally maintain an asymmetric distribution of phospholipids in the two leaflets of the plasma membrane bilayer. Alterations to this asymmetry, particularly the exposure of phosphatidylserine (PS) in the outer leaflet, can be recognised by phagocytes. Because of the importance of innate immune defence numerous studies have investigated PS exposure in RBCs infected with P. falciparum, but have reached different conclusions. Here we review recent advancements in our understanding of the molecular mechanisms which regulate asymmetry in RBCs, and whether infection with the P. falciparum parasite results in changes to PS exposure. On the balance of evidence, it is likely that membrane asymmetry is disrupted in parasitised RBCs, though some methodological issues need addressing. We discuss the potential causes and consequences of altered asymmetry in parasitised RBCs, particularly for in vivo interactions with the immune system, and the role of host-parasite co-evolution. We also examine the potential asymmetric state of parasite membranes and summarise current knowledge on the parasite proteins, which could regulate asymmetry in these membranes. Finally, we highlight unresolved questions at this time and the need for interdisciplinary approaches to uncover the machinery which enables P. falciparum parasites to hide in mature erythrocytes.




Similar content being viewed by others
References
Weatherall DJ (2008) Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol 141(3):276–286
Bretscher MS (1972) Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol 236(61):11–12
Gordesky SE, Marinetti GV (1973) The asymmetric arrangement of phospholipids in the human erythrocyte membrane. Biochem Biophys Res Commun 50(4):1027–1031
Lemmon MA (2008) Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9(2):99–111
Leventis PA, Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39(1):407–427
Kazanietz MG, Barchi JJ, Omichinski JG, Blumberg PM (1995) Low affinity binding of phorbol esters to protein kinase C and its recombinant cysteine-rich region in the absence of phospholipids. J Biol Chem 270(24):14679–14684
Mombers C, de Gier J, Demel RA, van Deenen LL (1980) Spectrin-phospholipid interaction. A monolayer study. Biochim Biophys Acta 603(1):52–62
An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N (2004) Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry 43(2):310–315
Manno S, Takakuwa Y, Mohandas N (2002) Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci USA 99(4):1943–1948
Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319(5860):210–213
Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, Van Schie RCAA, LaFace DM et al (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of BCL-2 and Abl. J Exp Med 182(5):1545–1556
McEvoy L, Williamson P, Schlegel RA (1986) Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci USA 83(10):3311–3315
Connor J, Pak C, Schroit A (1994) Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J Biol Chem 269:2399–2404
Fadok VA, De Cathelineau A, Daleke DL, Henson PM, Bratton DL (2001) Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem 276(2):1071–1077
Schwartz RS, Tanaka Y, Fidler IJ, Chiu DT, Lubin B, Schroit AJ (1985) Increased adherence of sickled and phosphatidylserine-enriched human erythrocytes to cultured human peripheral blood monocytes. J Clin Investig 75(6):1965–1972
Naeini MB, Bianconi V, Pirro M, Sahebkar A (2020) The role of phosphatidylserine recognition receptors in multiple biological functions. Cell Mol Bio Lett 25:23
Birge RB, Boeltz S, Kumar S, Carlson J, Wanderley J, Calianese D et al (2016) Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ 23(6):962–978
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73(4):1907–1916
Pretorius E, du Plooy JN, Bester J (2016) A comprehensive review on eryptosis. Cell Physiol Biochem 39(5):1977–2000
Lang E, Qadri SM, Lang F (2012) Killing me softly—suicidal erythrocyte death. Int J Biochem Cell 44(8):1236–1243
Pomorski T, Menon AK (2006) Lipid flippases and their biological functions. Cell Mol Life Sci 63(24):2908–2921
Daleke DL (2007) Phospholipid flippases. J Biol Chem 282(2):821–825
Hankins HM, Baldridge RD, Xu P, Graham TR (2015) Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 16(1):35–47
Zachowski A, Favre E, Cribier S, Hervé P, Devaux PF (1986) Outside-inside translocation of aminophospholipids in the human erythrocyte membrane is mediated by a specific enzyme. Biochemistry 25(9):2585–2590
Bitbol M, Devaux PF (1988) Measurement of outward translocation of phospholipids across human erythrocyte membrane. Proc Natl Acad Sci USA 85(18):6783–6787
Wang J, Molday LL, Hii T, Coleman JA, Wen T, Andersen JP et al (2018) Proteomic analysis and functional characterization of P4-ATPase phospholipid flippases from murine tissues. Sci Rep 8:10795
Andersen JP, Vestergaard AL, Mikkelsen SA, Mogensen LS, Chalat M, Molday RS (2016) P4-ATPases as phospholipid flippases—structure, function, and enigmas. Front Physiol 7:275
Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341(6144):403–406
Van Kruchten R, Mattheij NJA, Saunders C, Feijge MAH, Swieringa F, Wolfs JLN et al (2013) Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood 121(10):1850–1857
Bevers EM, Comfurius P, Zwaal RFA (1983) Changes in membrane phospholipid distribution during platelet activation. BBA Biomembr 736(1):57–66
Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF (1982) Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem 122(2):429–436
Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD et al (2009) Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 114(3):663–666
Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S (2014) Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344(6188):1164–1168
Arashiki N, Takakuwa Y (2019) Role of cholesterol in maintaining asymmetric distribution of phosphatidylserine in plasma membranes. In: Patel VB (ed) The molecular nutrition of fats. Academic Press, pp 77–86
Arashiki N, Saito M, Koshino I, Kamata K, Hale J, Mohandas N et al (2016) An unrecognized function of cholesterol: regulating the mechanism controlling membrane phospholipid asymmetry. Biochemistry 55(25):3504–3513
van Zwieten R, Bochem AE, Hilarius PM, Van Bruggen R, Kees Hovingh G, Verhoeven AJ (2012) The cholesterol content of the erythrocyte membrane is an important determinant of phosphatidylserine exposure. Biochim Biophys Acta 1821(12):1493–1500
Williamson P, Bateman J, Kozarsky K, Mattocks K, Hermanowicz N, Choe HR et al (1982) Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell 30(3):725–733
Kuypers F, Lewis R, Hua M, Schott M, Discher D, Ernst J et al (1996) Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood 87(3):1179–1187
Daleke DL (2008) Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol 15(3):191–195
Sathi A, Viswanad V, Aneesh TP, Kumar BA (2014) Pros and cons of phospholipid asymmetry in erythrocytes. J Pharm Bioallied Sci 6(2):81–85
Arese P, Turrini F, Schwarzer E (2005) Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell Physiol Biochem 16(4–6):133–146
Paulusma CC, Oude Elferink RPJ (2010) P4 ATPases—the physiological relevance of lipid flipping transporters. FEBS Lett 584(13):2708–2716
van der Mark VA, Elferink RPJO, Paulusma CC (2013) P4 ATPases: flippases in health and disease. Int J Mol Sci 14(4):7897–7922
Liou AY, Molday LL, Wang J, Andersen JP, Molday RS (2019) Identification and functional analyses of disease-associated P4-ATPase phospholipid flippase variants in red blood cells. J Biol Chem 294(17):6809–6821
Bryk AH, Wiśniewski JR (2017) Quantitative analysis of human red blood cell proteome. J Proteome Res 16(8):2752–2761
Arashiki N, Takakuwa Y, Mohandas N, Hale J, Yoshida K, Ogura H et al (2016) ATP11C is a major flippase in human erythrocytes and its defect causes congenital hemolytic anemia. Haematologica 101(5):559–565
Yabas M, Coupland LA, Cromer D, Winterberg M, Teoh NC, D’Rozario J et al (2014) Mice deficient in the putative phospholipid flippase ATP11C exhibit altered erythrocyte shape, anemia, and reduced erythrocyte life span. J Biol Chem 289(28):19531–19537
Yabas M, Teh CE, Frankenreiter S, Lal D, Roots CM, Whittle B et al (2011) ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol 12(5):441–449
Yabas M, Jing W, Shafik S, Bröer S, Enders A (2016) ATP11C facilitates phospholipid translocation across the plasma membrane of all leukocytes. PLoS ONE 11(1):e0146774
Quazi F, Molday RS (2013) Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem 288(48):34414–34426
Quazi F, Molday RS (2011) Lipid transport by mammalian ABC proteins. Essays Biochem 50(1):265–290
Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A (2003) Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2). Biochem J 376(2):489–495
Kang JH, Ko HM, Han GD, Lee SY, Moon JS, Kim MS et al (2020) Dual role of phosphatidylserine and its receptors in osteoclastogenesis. Cell Death Dis 11(7):497
van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P et al (1996) MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87(3):507–517
Kamp D, Haest CWM (1998) Evidence for a role of the multidrug resistance protein (MRP) in the outward translocation of NBD-phospholipids in the erythrocyte membrane. Biochim Biophys Acta Biomembr 1372(1):91–101
Pulaski L, Jedlitschky G, Leier I, Buchholz U, Keppler D (1996) Identification of the multidrug-resistance protein (MRP) as the glutathione-S-conjugate export pump of erythrocytes. Eur J Biochem 241(2):644–648
Dekkers DWC, Comfurius P, Van Gool RGJ, Bevers EM, Zwaal RFA (2000) Multidrug resistance protein 1 regulates lipid asymmetry in erythrocyte membranes. Biochem J 350(2):531–535
Neumann J, Rose-Sperling D, Hellmich UA (2017) Diverse relations between ABC transporters and lipids: an overview. Biochim Biophys Acta Biomembr 1859(4):605–618
Leimanis ML, Georges E (2007) ABCG2 membrane transporter in mature human erythrocytes is exclusively homodimer. Biochem Biophys Res Commun 354:345–350
Bassé F, Stout JG, Sims PJ, Wiedmer T (1996) Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem 271(29):17205–17210
Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ (1997) Molecular cloning of human plasma membrane phospholipid scramblase. J Biol Chem 272(29):18240–18244
Suzuki J, Imanishi E, Nagata S (2014) Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J Biol Chem 289(44):30257–30267
Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468(7325):834–840
Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S (2013) Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem 288(19):13305–13316
Wiedmer T, Zhou Q, Kwoh DY, Sims PJ (2000) Identification of three new members of the phospholipid scramblase gene family. Biochim Biophys Acta Biomembr 1467(1):244–253
Zhou Q, Zhao J, Wiedmer T, Sims PJ (2002) Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood 99(11):4030–4038
Bevers EM, Williamson PL (2010) Phospholipid scramblase: an update. FEBS Lett 584(13):2724–2730
Stout JG, Bassé F, Luhm RA, Weiss HJ, Wiedmer T, Sims PJ (1997) Scott syndrome erythrocytes contain a membrane protein capable of mediating Ca2+-dependent transbilayer migration of membrane phospholipids. J Clin Investig 99(9):2232–2238
Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L et al (2012) The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 120(15):e73-82
Zwaal RFA, Comfurius P, Bevers EM (2005) Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci 62(9):971–988
Bosman GJ (2004) Erythrocyte aging in sickle cell disease. Cell Mol Biol 50(1):81–86
Hannemann A, Rees DC, Brewin JN, Noe A, Low B, Gibson JS (2018) Oxidative stress and phosphatidylserine exposure in red cells from patients with sickle cell anaemia. Br J Haematol 182(4):567–578
Lubin B, Chiu D, Bastacky J, Roelofsen B, Van Deenen LL (1981) Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Investig 67(6):1643–1649
Kean LS, Brown LE, Nichols JW, Mohandas N, Archer DR, Hsu LL (2002) Comparison of mechanisms of anemia in mice with sickle cell disease and β-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp Hematol 30(5):394–402
Wanderley JLM, Damatta RA, Barcinski MA (2020) Apoptotic mimicry as a strategy for the establishment of parasitic infections: parasite- and host-derived phosphatidylserine as key molecule. Cell Commun Signal 18:10
Wanderley JLM, Moreira MEC, Benjamin A, Bonomo AC, Barcinski MA (2006) Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol 176(3):1834–1839
Balanco JMDF, Costa Moreira ME, Bonomo A, Bozza PT, Amarante-Mendes G, Pirmez C et al (2001) Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol 11(23):1870–1873
Mercer J, Helenius A (2008) Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320(5875):531–535
Grüring C, Moon RW, Lim C, Holder AA, Blackman MJ, Duraisingh MT (2014) Human red blood cell-adapted Plasmodium knowlesi parasites: a new model system for malaria research. Cell Microbiol 16(5):612–620
Kerlin DH, Gatton ML (2013) Preferential invasion by Plasmodium merozoites and the self-regulation of parasite burden. PLoS ONE 8(2):e57434
White N (2013) Malaria. In: Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White N (eds) Manson’s tropical diseases, 23rd edn. Elsevier Saunders, pp 532–600
Schwartz RS, Olson JA, Raventos-Suarez C, Yee M, Heath RH, Lubin B et al (1987) Altered plasma membrane phospholipid organization in Plasmodium falciparum-infected human erythrocytes. Blood 69(2):401–407
Maguire PA, Prudhomme J, Sherman IW (1991) Alterations in erythrocyte membrane phospholipid organization due to the intracellular growth of the human malaria parasite, Plasmodium falciparum. Parasitology 102(2):179–186
Joshi P, Gupta CM (1988) Abnormal membrane phospholipid organization in Plasmodium falciparum-infected human erythrocytes. Br J Haematol 68(2):255–259
Moll GN, Vial HJ, Bevers EM, Ancelin ML, Roelofsen B, Comfurius P et al (1990) Phospholipid asymmetry in the plasma membrane of malaria infected erythrocytes. Biochem Cell Biol 68:579–585
Subczynski WK, Pasenkiewicz-Gierula M, Widomska J, Mainali L, Raguz M (2017) High cholesterol/low cholesterol: effects in biological membranes: a review. Cell Biochem Biophys 75(3–4):369–385
Stillwell W (2016) Basic membrane properties of the fluid mosaic model. In: An introduction to biological membranes, 2nd edn, Chap 9. Elsevier, pp 135–180
Kirk K, Horner HA, Kirk J (1996) Glucose uptake in Plasmodium falciparum-infected erythrocytes is an equilibrative not an active process. Mol Biochem Parasitol 82(2):195–205
Preuss J, Jortzik E, Becker K (2012) Glucose-6-phosphate metabolism in Plasmodium falciparum. IUBMB Life 64(7):603–611
Roth EF Jr (1987) Malarial parasite hexokinase and hexokinase-dependent glutathione reduction in the Plasmodium falciparum-infected human erythrocyte. J Biol Chem 262(32):15678–15682
Roth EF (1990) Plasmodium falciparum carbohydrate metabolism: a connection between host cell and parasite. Blood Cells 16(2–3):453–460
Staines HM, Chang W, Ellory JC, Tiffert T, Kirk K, Lew VL (1999) Passive Ca2+ transport and Ca2+-dependent K+ transport in Plasmodium falciparum-infected red cells. J Membr Biol 172:13–24
Lew VL (1971) On the ATP dependence of the Ca2+-induced increase in K+ permeability observed in human red cells. Biochim Biophys Acta 233:827–830
Tiffert T, Bookchin RM, Lew VL (2003) Calcium homeostasis in normal and abnormal human red cells. In: Bernhardt I, Ellory JC (eds) Red cell membrane transport in health and disease. Springer, Berlin, pp 373–405
Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L (2013) Calcium in red blood cells—a perilous balance. Int J Mol Sci 14:9848–9872
Simões AP, Roelofsen B, Op den Kamp JAF (1992) Lipid compartmentalization in erythrocytes parasitized by Plasmodium spp. Parasitol Today 8(1):18–21
Taverne J, van Schie RCAA, Playfair JH, Reutelingsperger CPM (1996) Reply. Parasitol Today 12(3):122
Yen TC, Wey SP, Liao CH, Yeh CH, Shen DW, Achilefu S et al (2010) Measurement of the binding parameters of annexin derivative-erythrocyte membrane interactions. Anal Biochem 406(1):70–79
van Engeland M, Nieland LJW, Ramaekers FCS, Schutte B, Reutelingsperger CPM (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1–9
Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C (1995) A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184(1):39–51
Taverne J, van Schie R, Playfair J, Reutelingsperger C (1995) Malaria: phosphatidylserine expression is not increased on the surface of parasitized erythrocytes. Parasitol Today 11(8):298–299
Sherman I, Prudhomme J, Fair J (1997) Altered membrane phospholipid asymmetry in Plasmodium falciparum-infected erythrocytes. Parasitol Today 13(6):242–243
Ayi K, Giribaldi G, Skorokhod A, Schwarzer E, Prendergast PT, Arese P (2002) 16α-Bromoepiandrosterone, an antimalarial analogue of the hormone dehydroepiandrosterone, enhances phagocytosis of ring stage parasitized erythrocytes: a novel mechanism for antimalarial activity. Antimicrob Agents Chemother 46(10):3180–3184
Gelhaus C, Jacobs T, Andrä J, Leippe M (2008) The antimicrobial peptide NK-2, the core region of mammalian NK-lysin, kills intraerythrocytic Plasmodium falciparum. Antimicrob Agents Chemother 52(5):1713–1720
Eda S, Sherman IW (2002) Cytoadherence of malaria-infected red blood cells involves exposure of phosphatidylserine. Cell Physiol Biochem 12(5–6):373–384
Koka S, Huber SM, Boini KM, Lang C, Föller M, Lang F (2007) Lead decreases parasitemia and enhances survival of Plasmodium berghei-infected mice. Biochem Biophys Res Commun 363(3):484–489
Verhoeven AJ, Hilarius PM, Dekkers DWC, Lagerberg JWM, de Korte D (2006) Prolonged storage of red blood cells affects aminophospholipid translocase activity. Vox Sang 91(3):244–251
Romero PJ, Romero EA (1999) The role of calcium metabolism in human red blood cell ageing: a proposal. Blood Cells Mol Dis 25(1):9–19
Romero PJ, Romero EA (1997) Differences in Ca2+ pumping activity between sub-populations of human red cells. Cell Calcium 21(5):353–358
Lew VL, Daw N, Perdomo D, Etzion Z, Bookchin RM, Tiffert T (2003) Distribution of plasma membrane Ca2+ pump activity in normal human red blood cells. Blood 102(12):4206–4213
Bosman GJCGM, Cluitmans JCA, Groenen YAM, Werre JM, Willekens FLA, Novotný VMJ (2011) Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion 51(5):1072–1078
Boas FE, Forman L, Beutler E (1998) Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA 95(6):3077–3081
Dinkla S, Peppelman M, Der Raadt J, Atsma F, Novotńy VMJ, Van Kraaij MGJ et al (2014) Phosphatidylserine exposure on stored red blood cells as a parameter for donor-dependent variation in product quality. Blood Transfus 12(2):204–209
Zhang R, Chandramohanadas R, Lim CT, Dao M (2018) Febrile temperature elevates the expression of phosphatidylserine on Plasmodium falciparum (FCR3CSA) infected red blood cell surface leading to increased cytoadhesion. Sci Rep 8:15022
Pattanapanyasat K, Sratongno P, Chimma P, Chitjamnongchai S, Polsrila K, Chotivanich K (2010) Febrile temperature but not proinflammatory cytokines promotes phosphatidylserine expression on Plasmodium falciparum malaria-infected red blood cells during parasite maturation. Cytom Part A 77A(6):515–523
Engelbrecht D, Coetzer TL (2013) Turning up the heat: Heat stress induces markers of programmed cell death in Plasmodium falciparum in vitro. Cell Death Dis 4(12):e971
Brand V, Sandu C, Duranton C, Tanneur V, Lang K, Huber S et al (2003) Dependence of Plasmodium falciparum in vitro growth on the cation permeability of the human host erythrocyte. Cell Physiol Biochem 13(6):347–356
Bobbala D, Alesutan I, Föller M, Tschan S, Huber SM, Lang F (2010) Protective effect of amiodarone in malaria. Acta Trop 116(1):39–44
Engelbrecht D, Coetzer TL (2016) Plasmodium falciparum exhibits markers of regulated cell death at high population density in vitro. Parasitol Int 65:715–727
Fraser M, Jing W, Bröer S, Kurth F, Sander LE, Matuschewski K et al (2021) Breakdown in membrane asymmetry regulation leads to monocyte recognition of P. falciparum-infected red blood cells. PLoS Pathog 17(2):e1009259
Kasinathan RS, Föller M, Koka S, Huber SM, Lang F (2007) Inhibition of eryptosis and intraerythrocytic growth of Plasmodium falciparum by flufenamic acid. Naunyn Schmiedebergs Arch Pharmacol 374(4):255–264
Garcia CR, Dluzewski AR, Catalani LH, Burting R, Hoyland J, Mason WT (1996) Calcium homeostasis in intraerythrocytic malaria parasites. Eur J Cell Biol 71(4):409–413
Alleva LM, Kirk K (2001) Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 117(2):121–128
Rohrbach P, Friedrich O, Hentschel J, Plattner H, Fink RHA, Lanzer M (2005) Quantitative calcium measurements in subcellular compartments of Plasmodium falciparum-infected erythrocytes. J Biol Chem 280(30):27960–27969
Zipprer EM, Neggers M, Kushwaha A, Rayavara K, Desai SA (2014) A kinetic fluorescence assay reveals unusual features of Ca++ uptake in Plasmodium falciparum-infected erythrocytes. Malar J 13:184
Adovelande J, Bastide B, Délèze J, Schrével J (1993) Cytosolic free calcium in Plasmodium falciparum-infected erythrocytes and the effect of verapamil: a cytofluorimetric study. Exp Parasitol 76(3):247–258
Lang KS, Duranton C, Poehlmann H, Myssina S, Bauer C, Lang F et al (2003) Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ 10(2):249–256
Das BS, Nanda NK (1999) Evidence for erythrocyte lipid peroxidation in acute falciparum malaria. Trans R Soc Trop Med Hyg 93(1):58–62
Kulkarni AG, Suryakar AN, Sardeshmukh AS, Rathi DB (2003) Studies on biochemical changes with special reference to oxidant and antioxidants in malaria patients. Indian J Clin Biochem 18(2):136–149
Rashid MK, Alam R, Khan S, Prakash V (2013) Oxidative stress marker and antioxidant status in falciparum malaria in relation to the intensity of parasitaemia. Int J Biol Med Res 4(3):3469–3471
Kremsner PG, Greve B, Lell B, Luckner D, Schmid D (2000) Malarial anaemia in African children associated with high oxygen-radical production. Lancet 355(9197):40–41
Totino PRR, Magalhães A das D, Alves EB, Costa MRF, de Lacerda MVG, Daniel-Ribeiro CT et al (2014) Plasmodium falciparum, but not P vivax, can induce erythrocytic apoptosis. Parasite Vectors 7:484
Lang KS, Myssina S, Brand V, Sandu C, Lang PA, Berchtold S et al (2004) Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ 11(2):231–243
Abed M, Towhid ST, Mia S, Pakladok T, Alesutan I, Borst O et al (2012) Sphingomyelinase-induced adhesion of eryptotic erythrocytes to endothelial cells. Am J Physiol Cell Physiol 303(9):C991–C999
Lang E, Bissinger R, Gulbins E, Lang F (2015) Ceramide in the regulation of eryptosis, the suicidal erythrocyte death. Apoptosis 20(5):758–767
Aniweh Y, Gao X, Hao P, Meng W, Lai SK, Gunalan K et al (2017) P. falciparum RH5-Basigin interaction induces changes in the cytoskeleton of the host RBC. Cell Microbiol 19(9):e12747
Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA et al (2003) Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med 9(1):87–92
Lobo CA, Rodriguez M, Reid M, Lustigman S (2003) Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 101(11):4628–4631
Head DJ, Lee ZE, Poole J, Avent ND (2005) Expression of phosphatidylserine (PS) on wild-type and Gerbich variant erythrocytes following glycophorin-C (GPC) ligation. Br J Haematol 129(1):130–137
Boulet C, Doerig CD, Carvalho TG (2018) Manipulating eryptosis of human red blood cells: a novel antimalarial strategy? Front Cell Infect Microbiol 8:419
Ho M, Webster HK (1989) Immunology of human malaria. A cellular perspective. Parasite Immunol 11(2):105–116
Pongponratn E, Riganti M, Bunnag D, Harinasuta T (1987) Spleen in falciparum malaria: ultrastructural study. Southeast Asian J Trop Med Public Heal 18(4):491–501
Taliaferro WH, Mulligan H (1937) The histopathology of malaria with special reference to the function and origin of macrophages in defence. Indian Med Res Mem 29:1–138
Fendel R, Brandts C, Rudat A, Kreidenweiss A, Steur C, Appelmann I et al (2010) Hemolysis is associated with low reticulocyte production index and predicts blood transfusion in severe malarial anemia. PLoS ONE 5(4):e10038
Smith TG, Serghides L, Patel SN, Febbraio M, Silverstein RL, Kain KC (2003) CD36-mediated nonopsonic phagocytosis of erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum. Infect Immun 71(1):393–400
McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC (2000) Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96(9):3231–3240
Patel SN, Serghides L, Smith TG, Febbraio M, Silverstein RL, Kurtz TW et al (2004) CD36 mediates the phagocytosis of Plasmodium falciparum–infected erythrocytes by rodent macrophages. J Infect Dis 189(2):204–213
Turrini F, Ginsburg H, Bussolino F, Pescarmona GP, Serra MV, Arese P (1992) Phagocytosis of Plasmodium falciparum-infected human red blood cells by human monocytes: involvement of immune and nonimmune determinants and dependence on parasite developmental stage. Blood 80(3):801–808
Mikołajczyk TP, Skrzeczyńska-Moncznik JE, Zarȩbski MA, Marewicz EA, Wiśniewska AM, Dziȩba M et al (2009) Interaction of human peripheral blood monocytes with apoptotic polymorphonuclear cells. Immunology 128(1):103–113
Royo J, Rahabi M, Kamaliddin C, Ezinmegnon S, Olagnier D, Authier H et al (2019) Changes in monocyte subsets are associated with clinical outcomes in severe malarial anaemia and cerebral malaria. Sci Rep 9:17545
Facer CA, Agiostratidou G (1994) High levels of anti-phospholipid antibodies in uncomplicated and severe Plasmodium falciparum and in P. vivax malaria. Clin Exp Immunol 95(2):304–309
Owens S, Chamley LW, Ordi J, Brabin BJ, Johnson PM (2005) The association of anti-phospholipid antibodies with parity in placental malaria. Clin Exp Immunol 142(3):512–518
Fernandez-Arias C, Rivera-Correa J, Gallego-Delgado J, Rudlaff R, Fernandez C, Roussel C et al (2016) Anti-self phosphatidylserine antibodies recognize uninfected erythrocytes promoting malarial anemia. Cell Host Microbe 19(2):194–203
Rivera-Correa J, Mackroth MS, Jacobs T, Schulze zur Wiesch J, Rolling T, Rodriguez A (2019) Atypical memory B-cells are associated with Plasmodium falciparum anemia through anti-phosphatidylserine antibodies. eLife 8:e48309
Rivera-Correa J, Conroy AL, Opoka RO, Batte A, Namazzi R, Ouma B et al (2019) Autoantibody levels are associated with acute kidney injury, anemia and post-discharge morbidity and mortality in Ugandan children with severe malaria. Sci Rep 9:14940
Cunningham D, Lawton J, Jarra W, Preiser P, Langhorne J (2010) The pir multigene family of Plasmodium: antigenic variation and beyond. Mol Biochem Parasitol 170(2):65–73
Hall N, Karras M, Raine JD, Carlton JM, Kooij TWA, Berriman M et al (2005) A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307(5706):82–86
Carvalho BO, Lopes SCP, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC et al (2010) On the cytoadhesion of Plasmodium vivax–infected erythrocytes. J Infect Dis 202(4):638–647
Totino PR, Lopes SC (2017) Insights into the cytoadherence phenomenon of Plasmodium vivax: The putative role of phosphatidylserine. Front Immunol 8:1148
Aikawa M, Brown A, Smith CD, Tegoshi T, Howard RJ, Hasler TH et al (1992) A primate model for human cerebral malaria: Plasmodium coatneyi-infected rhesus monkeys. Am J Trop Med Hyg 46(4):391–397
Franke-Fayard B, Janse CJ, Cunha-Rodrigues M, Ramesar J, Büscher P, Que I et al (2005) Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci USA 102(32):11468–11473
Mota MM, Jarra W, Hirst E, Patnaik PK, Holder AA (2000) Plasmodium chabaudi-infected erythrocytes adhere to CD36 and bind to microvascular endothelial cells in an organ-specific way. Infect Immun 68(7):4135–4144
Franke-Fayard B, Fonager J, Braks A, Khan SM, Janse CJ (2010) Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria? PLoS Pathog 6(9):e1001032
Baker DA (2010) Malaria gametocytogenesis. Mol Biochem Parasitol 172(2):57–65
Hon C, Matuschewski K (2020) Malaria according to GARP: a new trail towards anti-disease vaccination. Trends Parasitol 36(8):653–655
Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A et al (2006) Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313(5791):1287–1290
Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U et al (2011) Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog 7(9):e1002224
Al-Olayan EM, Williams GT, Hurd H (2002) Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int J Parasitol 32(9):1133–1143
Arambage SC, Grant KM, Pardo I, Ranford-Cartwright L, Hurd H (2009) Malaria ookinetes exhibit multiple markers for apoptosis-like programmed cell death in vitro. Parasite Vectors 2:32
Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C (2015) Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci USA 112(5):1273–1280
Deponte M, Becker K (2004) Plasmodium falciparum—do killers commit suicide? Trends Parasitol 20(4):165–169
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW et al (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419(6906):498–511
Lamy A, Macarini-Bruzaferro E, Perálvarez-Marín A, le Maire M, Vázquez-Ibar JL (2021) ATP2, the essential P4-ATPase of malaria parasites, catalyzes lipid-dependent ATP hydrolysis in complex with a Cdc50 β-subunit. Emerg Microbes Infect 10(1):132–147
Kenthirapalan S, Waters AP, Matuschewski K, Kooij TWA (2016) Functional profiles of orphan membrane transporters in the life cycle of the malaria parasite. Nat Commun 7:10519
Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR et al (2018) Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360(6388):eaap7847
Bushell E, Gomes AR, Sanderson T, Anar B, Girling G, Herd C et al (2017) Functional profiling of a Plasmodium genome reveals an abundance of essential genes. Cell 170(2):260–272
Kavishe RA, Van Den Heuvel JM, Van De Vegte-Bolmer M, Luty AJ, Russel FG, Koenderink JB (2009) Localization of the ATP-binding cassette (ABC) transport proteins PfMRP1, PfMRP2, and PfMDR5 at the Plasmodium falciparum plasma membrane. Malar J 8:205
Raj DK, Mu J, Jiang H, Kabat J, Singh S, Sullivan M et al (2009) Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem 284(12):7687–7696
Klokouzas A, Tiffert T, Van Schalkwyk D, Wu CP, Van Veen HW, Barrand MA et al (2004) Plasmodium falciparum expresses a multidrug resistance-associated protein. Biochem Biophys Res Commun 321(1):197–201
Rubio JP, Cowman AF (1994) Plasmodium falciparum: the pfmdr2 protein is not overexpressed in chloroquine-resistant isolates of the malaria parasite. Exp Parasitol 79(2):137–147
Martin RE (2020) The transportome of the malaria parasite. Biol Rev 95(2):305–332
Haase S, Condron M, Miller D, Cherkaoui D, Jordan S, Gulbis JM et al (2020) Identification and characterisation of a phospholipid scramblase in the malaria parasite Plasmodium falciparum. https://doi.org/10.1101/2020.06.22.165258
Butthep P, Wanram S, Pattanapanyasat K, Vattanaviboon P, Fucharoen S, Wilairat P (2006) Cytoadherence between endothelial cells and P. falciparum infected and noninfected normal and thalassemic red blood cells. Cytom Part B Clin Cytom 70B(6):432–442
Koka S, Föller M, Lamprecht G, Boini KM, Lang C, Huber SM et al (2007) Iron deficiency influences the course of malaria in Plasmodium berghei infected mice. Biochem Biophys Res Commun 357(3):608–614
Niemoeller OM, Föller M, Lang C, Huber SM, Lang F (2008) Retinoic acid induced suicidal erythrocyte death. Cell Physiol Biochem 21:193–202
Koka S, Lang C, Niemoeller OM, Boini KM, Nicolay JP, Huber SM et al (2008) Influence of NO synthase inhibitor L-NAME on parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem 21(5–6):481–488
Koka S, Lang C, Boini K, Bobbala D, Huber S, Lang F (2008) Influence of chlorpromazine on eryptosis, parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem 22(1–4):261–268
Brand V, Koka S, Lang C, Jendrossek V, Huber SM, Gulbins E et al (2008) Influence of amitriptyline on eryptosis, parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem 22(5–6):405–412
Bobbala D, Koka S, Lang C, Boini KM, Huber SM, Lang F (2008) Effect of cyclosporine on parasitemia and survival of Plasmodium berghei infected mice. Biochem Biophys Res Commun 376(3):494–498
Koka S, Bobbala D, Lang C, Boini KM, Huber SM, Lang F (2009) Influence of paclitaxel on parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem 23:191–198
Bobbala D, Koka S, Geiger C, Föller M, Huber SM, Lang F (2009) Azathioprine favourably influences the course of malaria. Malar J 8:102
Lang PA, Kasinathan RS, Brand VB, Duranton C, Lang C, Koka S et al (2009) Accelerated clearance of Plasmodium-infected erythrocytes in sickle cell trait and Annexin-A7 deficiency. Cell Physiol Biochem 24(5–6):415–428
Alesutan I, Bobbala D, Qadri SM, Estremera A, Föller M, Lang F (2010) Beneficial effect of aurothiomalate on murine malaria. Malar J 9(1):118
Siraskar B, Ballal A, Bobbala D, Föller M, Lang F (2010) Effect of amphotericin B on parasitemia and survival of Plasmodium berghei-infected mice. Cell Physiol Biochem 26(3):347–354
Bobbala D, Alesutan I, Föller M, Huber SM, Lang F (2010) Effect of anandamide in Plasmodium berghei-infected mice. Cell Physiol Biochem 26(3):355–362
Ballal A, Bobbala D, Qadri SM, Föller M, Kempe D, Nasir O et al (2011) Anti-malarial effect of gum arabic. Malar J 10(1):139
Böttger E, Multhoff G, Kun JFJ, Esen M (2012) Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-Hsp70. PLoS ONE 7(3):e33774
Jiménez-Díaz MB, Ebert D, Salinas Y, Pradhan A, Lehane AM, Myrand-Lapierre ME et al (2014) (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc Natl Acad Sci USA 111(50):E5455–E5462
Acknowledgements
The authors would like to thank Andreas Herrmann for insightful discussions. This work was supported by the Alliance Berlin Canberra “Crossing Boundaries: Molecular Interactions in Malaria” which is co-funded by a grant from the Deutsche Forschungsgemeinschaft (DFG) for the International Research Training Group (IRTG) 2290 and the Australian National University. Work in the Maier group is also supported by the Australian Research Council (DP180103212). M.F. is the recipient of an Australian Government Research Training Program Scholarship. The authors declare that they have no conflict of interest.
Funding
Not applicable for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable for this study.
Ethics approval
Not applicable for this study.
Consent to participate
Not applicable for this study.
Consent for publication
Not applicable for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
18_2021_3799_MOESM1_ESM.docx
Table S1: Published studies which investigated membrane asymmetry in red blood cells infected with P. falciparum (iRBCs) and uninfected RBCs (uRBCs). Only results from RBCs not treated with modifying agent (drug, peptide, heat stress etc.) are shown. Percentages were calculated from tables or graphs using ImageJ where not specified in the text. PS phosphatidylserine; PE phosphatidylethanolamine; PC phosphatidylcholine; SM sphingomyelin; ATP adenosine triphosphate; enriched parasitaemias: increased by isolating iRBCs; effective parasitaemias: differentiated by DNA stain. (DOCX 30 KB)
Rights and permissions
About this article
Cite this article
Fraser, M., Matuschewski, K. & Maier, A.G. Of membranes and malaria: phospholipid asymmetry in Plasmodium falciparum-infected red blood cells. Cell. Mol. Life Sci. 78, 4545–4561 (2021). https://doi.org/10.1007/s00018-021-03799-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03799-6