Abstract
Adenosine is one of the most ancient signaling molecules and has receptors in both animals and plants. In mammals there are four specific receptors, A1, A2A, A2B, and A3, which belong to the superfamily of G-protein-coupled receptors (GPCRs). Evidence accumulated in the last 20 years indicates that GPCRs are often expressed as oligomeric complexes formed by a number of equal (homomers) or different (heteromers) receptors. This review presents the data showing the occurrence of heteromers formed by A1 and A2A, A2A and A2B, and A2A and A3 receptors highlighting (i) their tetrameric structural arrangements, and (ii) the functional diversity that those heteromers provide to adenosinergic signaling.

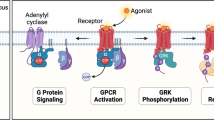
Adapted from reference


Adapted from reference

Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Burnstock G, Verkhratsky A (2009) Evolutionary origins of the purinergic signalling system [Internet]. Acta Physiol 195:415–447. https://doi.org/10.1111/j.1748-1716.2009.01957.x ((Wiley))
Alexander SP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017) The concise guide to pharmacology overview. Br J Pharmacol [Internet] 174:S1-16. https://doi.org/10.1111/bph.13882
Daly JW, Butts-Lamb P, Padgett W (1983) Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol [Internet] 3(1):69–80. https://doi.org/10.1007/BF00734999
Fredholm BB, Irenius E, Kull B, Schulte G (2001) Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61(4):443–448
Alnouri MW, Jepards S, Casari A, Schiedel AC, Hinz S, Müller CE (2015) Selectivity is species-dependent: characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal 11(3):389–407. https://doi.org/10.1007/s11302-015-9460-9
Hinz S, Alnouri WM, Pleiss U, Müller CE (2018) Tritium-labeled agonists as tools for studying adenosine A 2B receptors. Purinergic Signal [Internet]. 14(3):223–233. https://doi.org/10.1007/s11302-018-9608-5
Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB et al (1997) Comparative pharmacology of human adenosine receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol [Internet] 357(1):1–9. https://doi.org/10.1007/PL00005131
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full-length cDNAs from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A [Internet]. 85(23):8998–9002. Available from: https://pubmed.ncbi.nlm.nih.gov/2461560/
Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, et al. (1989) Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science [Internet]. 244(4904):569–72. Available from: https://science.sciencemag.org/content/244/4904/569
Jaakola V-P, Griffith MT, Hanson MA, Cherezov V, Chien EYT, Lane JR, et al. (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science [Internet]. 322(5905):1211–7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2586971&tool=pmcentrez&rendertype=abstract
Pándy-Szekeres G, Munk C, Tsonkov TM, Mordalski S, Harpsøe K, Hauser AS, et al. (2018) GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res [Internet]. 46(D1):D440–6. Available from: https://pubmed.ncbi.nlm.nih.gov/29155946/
Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jörg M, Scammells PJ, et al. (2017) Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell [Internet]. 168(5):867–877.e13. Available from: https://pubmed.ncbi.nlm.nih.gov/28235198/
Jespers W, Schiedel AC, Heitman LH, Cooke RM, Kleene L, van Westen GJP, et al. (2018) Structural Mapping of Adenosine Receptor Mutations: Ligand Binding and Signaling Mechanisms [Internet]. Trends Pharmacol Sci. 39: 75–89. Available from: https://pubmed.ncbi.nlm.nih.gov/29203139/
Drury AN, Szent-Györgyi A (1929) The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol [Internet]. 68(3):213–37. Available from: https://pubmed.ncbi.nlm.nih.gov/16994064/
Wolf MM, Berne RM (1956) Coronary vasodilator properties of purine and pyrimidine derivatives. Circ Res [Internet]. 4(3):343–348. https://doi.org/10.1161/01.RES.4.3.343
Buckley NM, Tsuboi KK, Zeig NJ (1959) Effect of nucleosides on acute left ventricular failure in the isolated dog heart. Circ Res [Internet] 7(6):847–857. https://doi.org/10.1161/01.RES.7.6.847
Agnati LF, Fuxe K, Ferri M, Benfenati F, Ogren SO (1981) A new hypothesis on memory—a possible role of local circuits in the formation of the memory trace. Med Biol [Internet]. 59(4):224–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7339294
Fuxe K, Agnati LF (1985) Receptor–receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med Res Rev [Internet]. 5(4):441–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2999530
Fuxe K, Agnati LF, Benfenati F, Cimmino M, Algeri S, Hökfelt T, et al. (1981) Modulation by cholecystokinins of 3 H-spiroperidol binding in rat striatum: evidence for increased affinity and reduction in the number of binding sites. Acta Physiol Scand [Internet]. 113(4):567–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6291324
Agnati LF, Fuxe K, Zoli M, Rondanini C, Ogren SO (1982) New vistas on synaptic plasticity: the receptor mosaic hypothesis of the engram. Med Biol [Internet]. 60(4):183–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6128444
Fuxe K, Härfstrand A, Agnati LF, Kalia M, Fredholm B, Svensson T, et al. (1987) Central catecholamine–neuropeptide Y interactions at the pre- and postsynaptic level in cardiovascular centers. J Cardiovasc Pharmacol [Internet]. 10 (Suppl 1):S1–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2455156
Fuxe K, Agnati LF, Benfenati F, Celani M, Zini I, Zoli M, et al. (1983) Evidence for the existence of receptor–receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J Neural Transm Suppl [Internet]. 18:165–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6192208
García-Recio A, Navarro G, Franco R, Olivella M, Guixà-González R, Cordomí A (2020) DIMERBOW: exploring possible GPCR dimer interfaces. Bioinformatics [Internet]. Available from: http://lmc.uab.es/dimerbow/.
Wu B, Chien EYT, Mol CD, Fenalti G, Liu W, Katritch V, et al. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science (80) [Internet]. 330(6007):1066–71. Available from: https://pubmed.ncbi.nlm.nih.gov/20929726/
Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature [Internet]. 485(7398):321–6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3523197&tool=pmcentrez&rendertype=abstract
Huang J, Chen S, Zhang JJ, Huang X-Y, Whorton MR, Bokoch MP, et al. (2013) Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol [Internet]. 20(4):419–25. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3618578&tool=pmcentrez&rendertype=abstract
Koehl A, Hu H, Feng D, Sun B, Zhang Y, Robertson MJ, et al. (2019) Structural insights into the activation of metabotropic glutamate receptors. Nature [Internet]. 566(7742):79–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30675062
Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin J-P, et al. (2002) A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA B receptor. J Biol Chem [Internet]. 277(5):3236–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11711539
Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin J-PJ (2011) A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J [Internet]. 25(1):66–77. Available from: https://pubmed.ncbi.nlm.nih.gov/20826542/
Rasmussen SGFF, Devree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS et al (2011) Crystal structure of the β 2 adrenergic receptor–Gs protein complex. Nature 477(7366):549–557
Navarro G, Gonzalez A, Campanacci S, Rivas-Santisteban R, Reyes-Resina I, Casajuana-Martin N, et al. (2020) Experimental and computational analysis of biased agonism on full-length and a C-terminally truncated adenosine A2A receptor. Comput Struct Biotechnol J [Internet]. 18:2723–32. Available from: https://pubmed.ncbi.nlm.nih.gov/33101610/
Draper-Joyce CJ, Khoshouei M, Thal DM, Liang YL, Nguyen ATN, Furness SGB, et al. (2018) Structure of the adenosine-bound human adenosine A1 receptor–Gi complex. Nature [Internet]. 558(7711):559–65. Available from: https://pubmed.ncbi.nlm.nih.gov/29925945/
Carpenter B, Nehmé R, Warne T, Leslie AGW, Tate CG (2016) Structure of the adenosine A2A receptor bound to an engineered G protein. Nature [Internet]. 536(7614):104–7. Available from: https://pubmed.ncbi.nlm.nih.gov/27462812/
García-Nafría J, Lee Y, Bai X, Carpenter B, Tate CG (2018) Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. Elife [Internet]. 7:e35946. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29726815
Viñals X, Moreno E, Lanfumey L, Cordomí A, Pastor A, de La Torre R, et al. (2015) Cognitive Impairment Induced by Delta9-tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB1 and Serotonin 5-HT2A Receptors. PLoS Biol [Internet]. 13(7):e1002194. Available from: https://pubmed.ncbi.nlm.nih.gov/26158621/
Guinart D, Moreno E, Galindo L, Cuenca-Royo A, Barrera-Conde M, Pérez EJ, et al. (2020) Altered signaling in CB1R-5-HT2AR heteromers in olfactory neuroepithelium cells of schizophrenia patients is modulated by cannabis use. Schizophr Bull [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/32249318/
Cordomí A, Navarro G, Aymerich MS, Franco R (2015) Structures for G-protein-coupled receptor tetramers in complex with G proteins. Trends Biochem Sci [Internet] 40(10):548–51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0968000415001280
Navarro G, Ferré S, Cordomi A, Moreno E, Mallol J, Casadó V, et al. (2010) Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J Biol Chem [Internet]. 285(35):27346–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20562103
Köfalvi A, Moreno E, Cordomí A, Cai NS, Fernández-Dueñas V, Ferreira SG, et al. (2020) Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biol [Internet]. 18(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31973708/
Whorton MR, Bokoch MP, Rasmussen SGF, Huang B, Zare RN, Kobilka B et al (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA 104(18):7682–7687
Navarro G, Cordomí A, Brugarolas M, Moreno E, Aguinaga D, Pérez-Benito L et al (2018) Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain. BMC Biol [Internet] 24(1):1–15. https://doi.org/10.1186/s12915-018-0491-x
Navarro G, Cordomí A, Zelman-Femiak M, Brugarolas M, Moreno E, Aguinaga D et al (2016) Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs. BMC Biol [Internet] 14(1):26. https://doi.org/10.1186/s12915-016-0247-4
Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA et al (2019) The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br J Pharmacol 1(176):S21-141
Borroto-Escuela DO, Brito I, Romero-Fernandez W, Di Palma M, Oflijan J, Skieterska K, et al. (2014) The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components. Int J Mol Sci [Internet]. 15(5):8570–90. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4057749&tool=pmcentrez&rendertype=abstract
Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KDG, Devi LA (2016) G protein-coupled receptor heteromers. Annu Rev Pharmacol Toxicol [Internet] 56(1):403–425. https://doi.org/10.1146/annurev-pharmtox-011613-135952
Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, et al. (2008) Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods [Internet]. 5(8):727–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18587404
Canals M, Burgueño J, Marcellino D, Cabello N, Canela EI, Mallol J et al (2004) Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Neurochem 88(3):726–734
Patowary S, Alvarez-Curto E, Xu TR, Holz JD, Oliver JA, Milligan G, et al. (2013) The muscarinic M3 acetylcholine receptor exists as two differently sized complexes at the plasma membrane. Biochem J [Internet]. 452(2):303–12. Available from: https://portlandpress.com/biochemj/article-pdf/452/2/303/676044/bj4520303.pdf
Gilchrist A, Li A, Hamm HE (2002) Design and use of C-terminal minigene vectors for studying role of heterotrimeric G proteins. In: Methods in Enzymology [Internet]. Academic Press Inc. p. 58–69. Available from: https://pubmed.ncbi.nlm.nih.gov/11771412/
Gilchrist A, Li A, Hamm H (2002) G alpha COOH-terminal minigene vectors dissect heterotrimeric G protein signaling. Sci STKE [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/11836477/
Guidotti G, Brambilla L, Rossi D (2017) Cell-Penetrating peptides: from basic research to clinics [Internet]. Vol. 38, Trends in pharmacological sciences. Elsevier Ltd; p. 406–24. Available from: https://pubmed.ncbi.nlm.nih.gov/28209404/
Milletti F (2012) Cell-penetrating peptides: classes, origin, and current landscape [Internet]. Vol. 17, Drug discovery today. Drug Discov Today. p. 850–60. Available from: https://pubmed.ncbi.nlm.nih.gov/22465171/
Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science (80) [Internet]. 285(5433):1569–72. Available from: https://pubmed.ncbi.nlm.nih.gov/10477521/
He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. (2011) Facilitation of μ-Opioid Receptor Activity by Preventing δ-Opioid Receptor-Mediated Codegradation. Neuron [Internet]. 69(1):120–31. Available from: https://pubmed.ncbi.nlm.nih.gov/21220103/
Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA (2000) Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci [Internet]. 20(22):RC110. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3125672&tool=pmcentrez&rendertype=abstract
Jastrzebska B, Chen Y, Orban T, Jin H, Hofmann L, Palczewski K (2015) Disruption of rhodopsin dimerization with synthetic peptides targeting an interaction interface. J Biol Chem [Internet] 290(42):25728–25744. https://doi.org/10.1074/jbc.M115.662684
Gnad T, Navarro G, Lahesmaa M, Reverte-Salisa L, Copperi F, Cordomi A, et al. (2020) Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metab [Internet]. 32(1). Available from: https://pubmed.ncbi.nlm.nih.gov/32589947/
Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M et al (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J [Internet] 27(17):2293–2304. https://doi.org/10.1038/emboj.2008.153
Xue L, Rovira X, Scholler P, Zhao H, Liu J, Pin J-P, et al. (2014) Major ligand-induced rearrangement of the heptahelical domain interface in a GPCR dimer. Nat Chem Biol [Internet]. 11(2):134–40. Available from: http://www.nature.com/articles/nchembio.1711
Caltabiano G, Gonzalez A, Cordomí A, Campillo M, Pardo L (2013) The role of hydrophobic amino acids in the structure and function of the rhodopsin family of G protein-coupled receptors. In: Methods in Enzymology [Internet]. Academic Press Inc.; p. 99–115. Available from: https://pubmed.ncbi.nlm.nih.gov/23332697/
Olivella M, Caltabiano G, Cordomí A (2013) The role of Cysteine 6.47 in class A GPCRs. BMC Struct Biol [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/23497259/
Ciruela F, Casadó V, Rodrigues R, Luján R, Burgueño J, Canals M et al (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A Receptor Heteromers. J Neurosci [Internet] 26(7):2080–2087. https://doi.org/10.1523/JNEUROSCI.3574-05.2006
Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, et al. (2013) A1R–A2AR heteromers coupled to Gs and G i/o proteins modulate GABA transport into astrocytes. Purinergic Signal [Internet]. 9(3):433–49. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3757138&tool=pmcentrez&rendertype=abstract
Hinz S, Navarro G, Borroto-Escuela D, Seibt BF, Ammon C, Filippo E De, et al. (2018) Adenosine A2A receptor ligand recognition and signaling is blocked by A2B receptors. Oncotarget [Internet]. 9(17):13593–611. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29568380
Gao ZG, Inoue A, Jacobson KA (2018) On the G protein-coupling selectivity of the native A2B adenosine receptor. Biochem Pharmacol 1(151):201–213
Yang X, Xin W, Yang X-M, Kuno A, Rich TC, Cohen MV et al (2011) A 2B adenosine receptors inhibit superoxide production from mitochondrial complex I in rabbit cardiomyocytes via a mechanism sensitive to Pertussis toxin. Br J Pharmacol [Internet] 163(5):995–1006. https://doi.org/10.1111/j.1476-5381.2011.01288.x
Gnad T, Scheibler S, Kugelgen I Von, Scheele C, Kilic A, Glode A, et al. (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature [Internet]. 516(7531):395–9. Available from: https://www.nature.com/articles/nature13816
Lahesmaa M, Oikonen V, Helin S, Luoto P, DinPfeifer UMA et al (2019) Regulation of human brown adipose tissue by adenosine and A 2A receptors—studies with [15 O]H 2 O and [11 C]TMSX PET/CT. Eur J Nucl Med Mol Imaging [Internet] 46(3):743–750. https://doi.org/10.1007/s00259-018-4120-2
Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB et al (2018) A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab 28(3):476-489.e5
Lillo A, Martínez-Pinilla E, Reyes-Resina I, Navarro G, Franco R (2020) Adenosine A2a and A3 receptors are able to interact with each other. A further piece in the puzzle of adenosine receptor-mediated signaling. Int J Mol Sci [Internet]. 21(14):1–14. Available from: https://www.mdpi.com/1422-0067/21/14/5070?utm_source=researcher_app&utm_medium=referral&utm_campaign=RESR_MRKT_Researcher_inbound
Hill SJ, May LT, Kellam B, Woolard J (2014) Allosteric interactions at adenosine A(1) and A(3) receptors: new insights into the role of small molecules and receptor dimerization. Br J Pharmacol [Internet]. 171(5):1102–13. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3952791&tool=pmcentrez&rendertype=abstract
Acknowledgements
Not applicable
Funding
This work was partially supported by grants SAF2017-84117, RTI2018-098830-B-I00, and PID2019-109240RB-I00 from the Spanish Ministry of Science and Innovation (MICINN) and or Science, Innovation and Universities; they may include EU FEDER funds. C.L.T. is the recipient of a FPI fellowship (BES-2017-081872). The laboratory of the University of Barcelona is considered of excellence by the Regional Catalonian Government (grup consolidat #2017 SGR 1497), which does not provide any specific funding for personnel, equipment and reagents or for payment of services.
Author information
Authors and Affiliations
Contributions
RF and LP conceived the idea and made a design of the sections in the review. AC, AL, and GN searched and summarized the content of relevant papers in literature. JSM and CLT looked information to prepare the Figs. RF wrote the first draft that was edited by all authors who also approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franco, R., Cordomí, A., Llinas del Torrent, C. et al. Structure and function of adenosine receptor heteromers. Cell. Mol. Life Sci. 78, 3957–3968 (2021). https://doi.org/10.1007/s00018-021-03761-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03761-6