Abstract
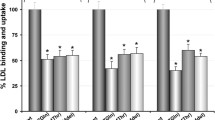
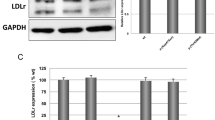
Naturally occurring point mutations in apolipoprotein A-I (apoA-I), the major protein component of high-density lipoprotein (HDL), may affect plasma HDL-cholesterol levels and cardiovascular risk. Here, we evaluated the effect of human apoA-I mutations L144R (associated with low HDL-cholesterol), L178P (associated with low HDL-cholesterol and increased cardiovascular risk) and A164S (associated with increased cardiovascular risk and mortality without low HDL-cholesterol) on the structural integrity and functions of lipid-free and lipoprotein-associated apoA-I in an effort to explain the phenotypes of subjects carrying these mutations. All three mutants, in lipid-free form, presented structural and thermodynamic aberrations, with apoA-I[L178P] presenting the greatest thermodynamic destabilization. Additionally, apoA-I[L178P] displayed reduced ABCA1-mediated cholesterol efflux capacity. When in reconstituted HDL (rHDL), apoA-I[L144R] and apoA-I[L178P] were more thermodynamically destabilized compared to wild-type apoA-I, both displayed reduced SR-BI-mediated cholesterol efflux capacity and apoA-I[L144R] showed severe LCAT activation defect. ApoA-I[A164S] was thermodynamically unaffected when in rHDL, but exhibited a series of functional defects. Specifically, it had reduced ABCG1-mediated cholesterol and 7-ketocholesterol efflux capacity, failed to reduce ROS formation in endothelial cells and had reduced capacity to induce endothelial cell migration. Mechanistically, the latter was due to decreased capacity of rHDL-apoA-I[A164S] to activate Akt kinase possibly by interacting with endothelial LOX-1 receptor. The impaired capacity of rHDL-apoA-I[A164S] to preserve endothelial function may be related to the increased cardiovascular risk for this mutation. Overall, our structure–function analysis of L144R, A164S and L178P apoA-I mutants provides insights on how HDL-cholesterol levels and/or atheroprotective properties of apoA-I/HDL are impaired in carriers of these mutations.













Similar content being viewed by others
Abbreviations
- ABCA1:
-
ATP-binding cassette A1
- ABCG1:
-
ATP-binding cassette G1
- ANS:
-
8-anilino-1-naphthalenesulfonic acid
- apoA-I:
-
Apolipoprotein A-I
- C:
-
Cholesterol
- CD:
-
Circular dichroism
- cpt-cAMP:
-
8-(4-Chlorophenylthio)-cAMP
- CVD:
-
Cardiovascular disease
- DCF:
-
2′,7′-Dichlorofluorescein
- DCFH-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- DPBS:
-
Dulbecco’s phosphate-buffered saline
- GdnHCl:
-
Guanidine hydrochloride
- HCAEC:
-
Human coronary artery endothelial cells
- HDL:
-
High-density lipoprotein
- HDL-C:
-
HDL-cholesterol
- IHD:
-
Ischemic heart disease
- IMT:
-
Intima–media thickness
- LCAT:
-
Lecithin:cholesterol acyltransferase
- LOX-1:
-
Lectin-type oxidized low-density lipoprotein receptor 1
- MDA:
-
Malondialdehyde
- Ni-NTA:
-
Ni2+-nitrilotriacetic acid
- oxPAPC:
-
Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- POPC:
-
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- rHDL:
-
Reconstituted HDL
- ROS:
-
Reactive oxygen species
- SR-BI:
-
Scavenger receptor class B type I
- TBARS:
-
Thiobarbituric acid reactive substances
- Trx:
-
Thioredoxin
- WT:
-
Wild type
References
Zannis VI, Fotakis P, Koukos G, Kardassis D, Ehnholm C, Jauhiainen M, Chroni A (2015) HDL biogenesis, remodeling, and catabolism. Handb Exp Pharmacol 224:53–111
Siddiqi HK, Kiss D, Rader D (2015) HDL-cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol 30:536–542
Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M (1996) Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518–520
Daniil G, Zannis VI, Chroni A (2013) Effect of apoA-I mutations in the capacity of reconstituted HDL to promote ABCG1-mediated cholesterol efflux. PLoS One 8:e67993
Mineo C, Shaul PW (2012) Novel biological functions of high-density lipoprotein cholesterol. Circ Res 111:1079–1090
Marz W, Kleber ME, Scharnagl H, Speer T, Zewinger S, Ritsch A, Parhofer KG et al (2017) HDL cholesterol: reappraisal of its clinical relevance. Clin Res Cardiol 106:663–675
Favari E, Thomas MJ, Sorci-Thomas MG (2018) High-density lipoprotein functionality as a new pharmacological target on cardiovascular disease: unifying mechanism that explains high-density lipoprotein protection toward the progression of atherosclerosis. J Cardiovasc Pharmacol 71:325–331
Melchior JT, Walker RG, Cooke AL, Morris J, Castleberry M, Thompson TB, Jones MK et al (2017) A consensus model of human apolipoprotein A-I in its monomeric and lipid-free state. Nat Struct Mol Biol 24:1093–1099
Mei X, Atkinson D (2011) Crystal structure of C-terminal truncated apolipoprotein A-I reveals the assembly of high density lipoprotein (HDL) by dimerization. J Biol Chem 286:38570–38582
Mei X, Atkinson D (2015) Lipid-free apolipoprotein A-I structure: insights into HDL formation and atherosclerosis development. Arch Med Res 46:351–360
Borhani DW, Rogers DP, Engler JA, Brouillette CG (1997) Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc Natl Acad Sci USA 94:12291–12296
Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A et al (2008) Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc Natl Acad Sci USA 105:12176–12181
Huang R, Silva RA, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ et al (2011) Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol 18:416–422
Gursky O (2013) Crystal structure of Delta(185-243)ApoA-I suggests a mechanistic framework for the protein adaptation to the changing lipid load in good cholesterol: from flatland to sphereland via double belt, belt buckle, double hairpin and trefoil/tetrafoil. J Mol Biol 425:1–16
Sorci-Thomas MG, Thomas MJ (2002) The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc Med 12:121–128
Zannis VI, Chroni A, Krieger M (2006) Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med 84:276–294
Chroni A, Kardassis D (2019) HDL dysfunction caused by mutations in apoA-I and other genes that are critical for HDL biogenesis and remodeling. Curr Med Chem 26:1544–1575
Haase CL, Frikke-Schmidt R, Nordestgaard BG, Kateifides AK, Kardassis D, Nielsen LB, Andersen CB et al (2011) Mutation in APOA1 predicts increased risk of ischaemic heart disease and total mortality without low HDL cholesterol levels. J Intern Med 270:136–146
Recalde D, Cenarro A, Garcia-Otin AL, Gomez-Coronado D, Civeira F, Pocovi M (2002) Analysis of apolipoprotein A-I, lecithin:cholesterol acyltransferase and glucocerebrosidase genes in hypoalphalipoproteinemia. Atherosclerosis 163:49–58
Hovingh GK, Brownlie A, Bisoendial RJ, Dube MP, Levels JH, Petersen W, Dullaart RP et al (2004) A novel apoA-I mutation (L178P) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol 44:1429–1435
Gkolfinopoulou C, Bourtsala A, Chroni A (2020) Structural and functional basis for increased HDL-cholesterol levels due to the naturally occurring V19L mutation in human apolipoprotein A-I. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158593
Argyri L, Skamnaki V, Stratikos E, Chroni A (2011) A simple approach for human recombinant apolipoprotein E4 expression and purification. Protein Expr Purif 79:251–257
Fotakis P, Tiniakou I, Kateifides AK, Gkolfinopoulou C, Chroni A, Stratikos E, Zannis VI et al (2013) Significance of the hydrophobic residues 225–230 of apoA-I for the biogenesis of HDL. J Lipid Res 54:3293–3302
Morrisett JD, David JS, Pownall HJ, Gotto AM Jr (1973) Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry 12:1290–1299
Gorshkova IN, Liadaki K, Gursky O, Atkinson D, Zannis VI (2000) Probing the lipid-free structure and stability of apolipoprotein A-I by mutation. Biochemistry 39:15910–15919
Chroni A, Liu T, Gorshkova I, Kan HY, Uehara Y, von Eckardstein A, Zannis VI (2003) The central helices of apoA-I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux. Amino acid residues 220-231 of the wild-type apoA-I are required for lipid efflux in vitro and high density lipoprotein formation in vivo. J Biol Chem 278:6719–6730
Dafnis I, Raftopoulou C, Mountaki C, Megalou E, Zannis VI, Chroni A (2018) ApoE isoforms and carboxyl-terminal-truncated apoE4 forms affect neuronal BACE1 levels and Abeta production independently of their cholesterol efflux capacity. Biochem J 475:1839–1859
Chadwick AC, Sahoo D (2012) Functional characterization of newly-discovered mutations in human SR-BI. PLoS One 7:e45660
Lambert G, Chase MB, Dugi K, Bensadoun A, Brewer HB Jr, Santamarina-Fojo S (1999) Hepatic lipase promotes the selective uptake of high density lipoprotein-cholesteryl esters via the scavenger receptor B1. J Lipid Res 40:1294–1303
Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC, Jessup W et al (2007) Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J Biol Chem 282:2851–2861
Chroni A, Kan HY, Kypreos KE, Gorshkova IN, Shkodrani A, Zannis VI (2004) Substitutions of glutamate 110 and 111 in the middle helix 4 of human apolipoprotein A-I (apoA-I) by alanine affect the structure and in vitro functions of apoA-I and induce severe hypertriglyceridemia in apoA-I-deficient mice. Biochemistry 43:10442–10457
Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD et al (2006) High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res 98:63–72
Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D et al (2013) Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 128:2026–2038
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Dafnis I, Argyri L, Sagnou M, Tzinia A, Tsilibary EC, Stratikos E, Chroni A (2016) The ability of apolipoprotein E fragments to promote intraneuronal accumulation of amyloid beta peptide 42 is both isoform and size-specific. Sci Rep 6:30654
Gursky O, Atkinson D (1996) Thermal unfolding of human high-density apolipoprotein A-1: implications for a lipid-free molten globular state. Proc Natl Acad Sci USA 93:2991–2995
Zhang X, Lei D, Zhang L, Rames M, Zhang S (2015) A model of lipid-free apolipoprotein A-I revealed by iterative molecular dynamics simulation. PLoS One 10:e0120233
Gorshkova IN, Liu T, Kan HY, Chroni A, Zannis VI, Atkinson D (2006) Structure and stability of apolipoprotein A-I in solution and in discoidal high-density lipoprotein probed by double charge ablation and deletion mutation. Biochemistry 45:1242–1254
Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL (2000) The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem 275:28634–28640
Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH (2004) Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol 24:2345–2350
Hafiane A, Genest J (2015) HDL-mediated cellular cholesterol efflux assay method. Ann Clin Lab Sci 45:659–668
Besler C, Luscher TF, Landmesser U (2012) Molecular mechanisms of vascular effects of High-density lipoprotein: alterations in cardiovascular disease. EMBO Mol Med 4:251–268
Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T et al (2008) ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Investig 118:3701–3713
Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A et al (2011) Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Investig 121:2693–2708
Soumyarani VS, Jayakumari N (2014) Oxidized HDL induces cytotoxic effects: implications for atherogenic mechanism. J Biochem Mol Toxicol 28:481–489
Solati Z, Ravandi A (2019) Lipidomics of bioactive lipids in acute coronary syndromes. Int J Mol Sci 20:E1051
Recalde D, Velez-Carrasco W, Civeira F, Cenarro A, Gomez-Coronado D, Ordovas JM, Pocovi M (2001) Enhanced fractional catabolic rate of apo A-I and apo A-II in heterozygous subjects for apo A-I(Zaragoza) (L144R). Atherosclerosis 154:613–623
Daniil G, Phedonos AA, Holleboom AG, Motazacker MM, Argyri L, Kuivenhoven JA, Chroni A (2011) Characterization of antioxidant/anti-inflammatory properties and apoA-I-containing subpopulations of HDL from family subjects with monogenic low HDL disorders. Clin Chim Acta 412:1213–1220
Dalla-Riva J, Lagerstedt JO, Petrlova J (2015) Structural and functional analysis of the apolipoprotein A-I A164S variant. PLoS One 10:e0143915
Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL et al (2005) Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Investig 115:969–977
Shi Y, Lv X, Liu Y, Li B, Liu M, Yan M, Liu Y et al (2018) Elevating ATP-binding cassette transporter G1 improves re-endothelialization function of endothelial progenitor cells via Lyn/Akt/eNOS in diabetic mice. FASEB J 32:6525–6536
Marsche G, Levak-Frank S, Quehenberger O, Heller R, Sattler W, Malle E (2001) Identification of the human analog of SR-BI and LOX-1 as receptors for hypochlorite-modified high density lipoprotein on human umbilical venous endothelial cells. FASEB J 15:1095–1097
Acknowledgements
We thank Dr. Dimitris Kardassis (Medical School of the University of Crete, Greece) and members of his lab for providing valuable help with the endothelial cell migration assay. We acknowledge support of this work by the projects: (a) “An Open-Access Research Infrastructure of Chemical Biology and Target-Based Screening Technologies for Human and Animal Health, Agriculture and the Environment (OPENSCREEN-GR)” (MIS 5002691) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund), (b) “The National Research Infrastructure on Integrated Structural Biology, Drug Screening Efforts and Drug target functional characterization (INSPIRED)” (MIS 5002550) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund), (c) “A Greek Research Infrastructure for Visualizing and Monitoring Fundamental Biological Processes (BioImaging-GR)” (MIS 5002755) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund) and (d) “CURE-PLaN” a grant from the Leducq Foundation for Cardiovascular Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gkolfinopoulou, C., Soukou, F., Dafnis, I. et al. Structure–function analysis of naturally occurring apolipoprotein A-I L144R, A164S and L178P mutants provides insight on their role on HDL levels and cardiovascular risk. Cell. Mol. Life Sci. 78, 1523–1544 (2021). https://doi.org/10.1007/s00018-020-03583-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03583-y