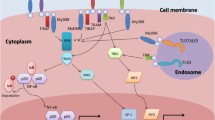
Abstract
B cells mediate humoral immune response and contribute to the regulation of cellular immune response. Members of the Nuclear Factor kappaB (NF-κB) family of transcription factors play a major role in regulating B-cell functions. NF-κB subunit c-Rel is predominantly expressed in lymphocytes, and in B cells, it is required for survival, proliferation, and antibody production. Dysregulation of c-Rel expression and activation alters B-cell homeostasis and is associated with B-cell lymphomas and autoimmune pathologies. Based on its essential roles, c-Rel may serve as a potential prognostic and therapeutic target. This review summarizes the current understanding of the multifaceted role of c-Rel in B cells and B-cell diseases.


Similar content being viewed by others
References
Sen R, Baltimore D (1986) Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47:921–928
Pomerantz JL, Baltimore D (2002) Two pathways to NF-kappaB. Mol Cell 10:693–695
Ramakrishnan P, Wang W, Wallach D (2004) Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity 21:477–489. https://doi.org/10.1016/j.immuni.2004.08.009
Oeckinghaus A, Ghosh S (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1:a000034. https://doi.org/10.1101/cshperspect.a000034
Gilmore TD (1991) Malignant transformation by mutant Rel proteins. Trends Genet 7:318–322. https://doi.org/10.1016/0168-9525(91)90421-l
Doerre S, Sista P, Sun SC et al (1993) The c-rel protooncogene product represses NF-kappa B p65-mediated transcriptional activation of the long terminal repeat of type 1 human immunodeficiency virus. Proc Natl Acad Sci USA 90:1023–1027
Sohur US, Dixit MN, Chen C-L et al (1999) Rel/NF-κB represses bcl-2 transcription in pro-B lymphocytes. Gene Expr 8:219–229
Roy K, Mitchell S, Liu Y et al (2019) A Regulatory circuit controlling the dynamics of NFκB cRel transitions B cells from proliferation to plasma cell differentiation. Immunity 50:616–628.e6. https://doi.org/10.1016/j.immuni.2019.02.004
de Jesús TJ, Centore JT, Ramakrishnan P (2019) Differential regulation of basal expression of inflammatory genes by NF-κB family subunits. Cell Mol Immunol 16:720–723. https://doi.org/10.1038/s41423-019-0242-0
Fullard N, Wilson CL, Oakley F (2012) Roles of c-Rel signalling in inflammation and disease. Int J Biochem Cell Biol 44:851–860. https://doi.org/10.1016/j.biocel.2012.02.017
Glineur C, Davioud-Charvet E, Vandenbunder B (2000) The conserved redox-sensitive cysteine residue of the DNA-binding region in the c-Rel protein is involved in the regulation of the phosphorylation of the protein. Biochem J 352:583–591
Chen E, Hrdlickova R, Nehyba J et al (1998) Degradation of proto-oncoprotein c-Rel by the ubiquitin-proteasome pathway. J Biol Chem 273:35201–35207. https://doi.org/10.1074/jbc.273.52.35201
Leeman JR, Weniger MA, Barth TF, Gilmore TD (2008) Deletion analysis and alternative splicing define a transactivation inhibitory domain in human oncoprotein REL. Oncogene 27:6770–6781. https://doi.org/10.1038/onc.2008.284
Huang DB, Chen YQ, Ruetsche M et al (2001) X-ray crystal structure of proto-oncogene product c-Rel bound to the CD28 response element of IL-2. Structure 9:669–678. https://doi.org/10.1016/s0969-2126(01)00635-9
Ramakrishnan P, Clark PM, Mason DE et al (2013) Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Sci Signal 6:ra75. https://doi.org/10.1126/scisignal.2004097
Martin AG, Fresno M (2000) Tumor necrosis factor-alpha activation of NF-kappa B requires the phosphorylation of Ser-471 in the transactivation domain of c-Rel. J Biol Chem 275:24383–24391. https://doi.org/10.1074/jbc.M909396199
Fognani C, Rondi R, Romano A, Blasi F (2000) cRel-TD kinase: a serine/threonine kinase binding in vivo and in vitro c-Rel and phosphorylating its transactivation domain. Oncogene 19:2224–2232. https://doi.org/10.1038/sj.onc.1203543
Harris J, Olière S, Sharma S et al (2006) Nuclear accumulation of cRel following C-terminal phosphorylation by TBK1/IKK epsilon. J Immunol 177:2527–2535. https://doi.org/10.4049/jimmunol.177.4.2527
Köntgen F, Grumont RJ, Strasser A et al (1995) Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev 9:1965–1977. https://doi.org/10.1101/gad.9.16.1965
Tumang JR, Owyang A, Andjelic S et al (1998) c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur J Immunol 28:4299–4312. https://doi.org/10.1002/(SICI)1521-4141(199812)28:12%3c4299:AID-IMMU4299%3e3.0.CO;2-Y
Gerondakis S, Grossmann M, Nakamura Y et al (1999) Genetic approaches in mice to understand Rel/NF-kappaB and IkappaB function: transgenics and knockouts. Oncogene 18:6888–6895. https://doi.org/10.1038/sj.onc.1203236
Yamazaki T, Kurosaki T (2003) Contribution of BCAP to maintenance of mature B cells through c-Rel. Nat Immunol 4:780–786. https://doi.org/10.1038/ni949
Matsuda S, Mikami Y, Ohtani M et al (2009) Critical role of class IA PI3K for c-Rel expression in B lymphocytes. Blood 113:1037–1044. https://doi.org/10.1182/blood-2008-06-163725
Hu CJ, Rao S, Ramirez-Bergeron DL et al (2001) PU.1/Spi-B regulation of c-rel is essential for mature B-cell survival. Immunity 15:545–555
Cho S, Lee H-M, Yu I-S et al (2018) Differential cell-intrinsic regulations of germinal center B and T cells by miR-146a and miR-146b. Nat Commun 9:2757. https://doi.org/10.1038/s41467-018-05196-3
Pieper K, Grimbacher B, Eibel H (2013) B-cell biology and development. J Allergy Clin Immunol 131:959–971. https://doi.org/10.1016/j.jaci.2013.01.046
Gilmore TD, Gerondakis S (2011) The c-Rel transcription factor in development and disease. Genes Cancer 2:695–711. https://doi.org/10.1177/1947601911421925
Ramakrishnan P, Yui MA, Tomalka JA et al (2016) Deficiency of nuclear factor-κB c-Rel accelerates the development of autoimmune diabetes in NOD mice. Diabetes 65:2367–2379. https://doi.org/10.2337/db15-1607
Grumont RJ, Gerondakis S (1994) The subunit composition of NF-kappa B complexes changes during B-cell development. Cell Growth Differ 5:1321–1331
Vazquez MI, Catalan-Dibene J, Zlotnik A (2015) B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 74:318–326. https://doi.org/10.1016/j.cyto.2015.02.007
Yam-Puc JC, Zhang L, Zhang Y, Toellner K-M (2018) Role of B-cell receptors for B-cell development and antigen-induced differentiation. F1000Res 7:429. https://doi.org/10.12688/f1000research.13567.1
Grumont RJ, Rourke IJ, O’Reilly LA et al (1998) B lymphocytes differentially use the Rel and nuclear factor kappaB1 (NF-kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J Exp Med 187:663–674. https://doi.org/10.1084/jem.187.5.663
Hsia CY, Cheng S, Owyang AM et al (2002) c-Rel regulation of the cell cycle in primary mouse B lymphocytes. Int Immunol 14:905–916. https://doi.org/10.1093/intimm/dxf055
Pohl T, Gugasyan R, Grumont RJ et al (2002) The combined absence of NF-kappa B1 and c-Rel reveals that overlapping roles for these transcription factors in the B-cell lineage are restricted to the activation and function of mature cells. Proc Natl Acad Sci USA 99:4514–4519. https://doi.org/10.1073/pnas.072071599
Grossmann M, O’Reilly LA, Gugasyan R et al (2000) The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J 19:6351–6360. https://doi.org/10.1093/emboj/19.23.6351
Grumont RJ, Gerondakis S (2000) Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med 191:1281–1292. https://doi.org/10.1084/jem.191.8.1281
Cheng S, Hsia CY, Leone G, Liou H-C (2003) Cyclin E and Bcl-xL cooperatively induce cell cycle progression in c-Rel−/− B cells. Oncogene 22:8472–8486. https://doi.org/10.1038/sj.onc.1206917
Gugasyan R, Grumont R, Grossmann M et al (2000) Rel/NF-kappaB transcription factors: key mediators of B-cell activation. Immunol Rev 176:134–140
Shukla V, Lu R (2014) IRF4 and IRF8: governing the virtues of B lymphocytes. Front Biol (Beijing) 9:269–282. https://doi.org/10.1007/s11515-014-1318-y
Grumont RJ, Rourke IJ, Gerondakis S (1999) Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev 13:400–411
Banerjee A, Grumont R, Gugasyan R et al (2008) NF-κB1 and c-Rel cooperate to promote the survival of TLR4-activated B cells by neutralizing Bim via distinct mechanisms. Blood 112:5063–5073. https://doi.org/10.1182/blood-2007-10-120832
Owyang AM, Tumang JR, Schram BR et al (2001) c-Rel is required for the protection of B cells from antigen receptor-mediated, but not Fas-mediated, apoptosis. J Immunol 167:4948–4956. https://doi.org/10.4049/jimmunol.167.9.4948
Shawgo ME, Shelton SN, Robertson JD (2008) Caspase-mediated Bak activation and cytochrome c release during intrinsic apoptotic cell death in jurkat cells. J Biol Chem 283:35532–35538. https://doi.org/10.1074/jbc.M807656200
Castro I, Wright JA, Damdinsuren B et al (2009) B-cell receptor-mediated sustained c-Rel activation facilitates late transitional B-cell survival through control of B-cell activating factor receptor and NF-κB2. J Immunol 182:7729–7737. https://doi.org/10.4049/jimmunol.0803281
Heise N, De Silva NS, Silva K et al (2014) Germinal center B-cell maintenance and differentiation are controlled by distinct NF-κB transcription factor subunits. J Exp Med 211:2103–2118. https://doi.org/10.1084/jem.20132613
De Silva NS, Anderson MM, Carette A et al (2016) Transcription factors of the alternative NF-κB pathway are required for germinal center B-cell development. Proc Natl Acad Sci USA 113:9063–9068. https://doi.org/10.1073/pnas.1602728113
Carrasco D, Cheng J, Lewin A et al (1998) Multiple hemopoietic defects and lymphoid hyperplasia in mice lacking the transcriptional activation domain of the c-Rel protein. J Exp Med 187:973–984. https://doi.org/10.1084/jem.187.7.973
Casola S, Cattoretti G, Uyttersprot N et al (2006) Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc Natl Acad Sci USA 103:7396–7401. https://doi.org/10.1073/pnas.0602353103
Neo WH, Lim JF, Grumont R et al (2014) c-Rel regulates Ezh2 expression in activated lymphocytes and malignant lymphoid cells. J Biol Chem 289:31693–31707. https://doi.org/10.1074/jbc.M114.574517
Caganova M, Carrisi C, Varano G et al (2013) Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J Clin Investig 123:5009–5022. https://doi.org/10.1172/JCI70626
Boothby M, Rickert RC (2017) Metabolic regulation of the immune humoral response. Immunity 46:743–755. https://doi.org/10.1016/j.immuni.2017.04.009
Calado DP, Sasaki Y, Godinho SA et al (2012) The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol 13:1092–1100. https://doi.org/10.1038/ni.2418
Dominguez-Sola D, Victora GD, Ying CY et al (2012) The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 13:1083–1091. https://doi.org/10.1038/ni.2428
Dang CV (1999) c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol 19:1–11. https://doi.org/10.1128/mcb.19.1.1
Gerondakis S, Siebenlist U (2010) Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol 2:a000182. https://doi.org/10.1101/cshperspect.a000182
Klein U, Heise N (2015) Unexpected functions of NFκB during germinal center B-cell development: implications for lymphomagenesis. Curr Opin Hematol 22:379–387. https://doi.org/10.1097/MOH.0000000000000160
Kennedy R, Klein U (2018) Aberrant activation of NF-κB signalling in aggressive lymphoid malignancies. Cells 7:189. https://doi.org/10.3390/cells7110189
Taplin CE, Barker JM (2008) Autoantibodies in type 1 diabetes. Autoimmunity 41:11–18. https://doi.org/10.1080/08916930701619169
Maurer M, Altrichter S, Schmetzer O et al (2018) Immunoglobulin E-mediated autoimmunity. Front Immunol 9:689. https://doi.org/10.3389/fimmu.2018.00689
Huijbers MG, Plomp JJ, van der Maarel SM, Verschuuren JJ (2018) IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Ann N Y Acad Sci 1413:92–103. https://doi.org/10.1111/nyas.13561
Ballow M (2002) Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol 109:581–591. https://doi.org/10.1067/mai.2002.122466
Fried AJ, Bonilla FA (2009) Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin Microbiol Rev 22:396–414. https://doi.org/10.1128/CMR.00001-09
Kenter AL (2003) Class-switch recombination: after the dawn of AID. Curr Opin Immunol 15:190–198
Sleckman BP, Gorman JR, Alt FW (1996) Accessibility control of antigen-receptor variable-region gene assembly: role of cis-acting elements. Annu Rev Immunol 14:459–481. https://doi.org/10.1146/annurev.immunol.14.1.459
Reimold AM, Iwakoshi NN, Manis J et al (2001) Plasma cell differentiation requires the transcription factor XBP-1. Nature 412:300–307. https://doi.org/10.1038/35085509
Shapiro-Shelef M, Lin K-I, McHeyzer-Williams LJ et al (2003) Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19:607–620. https://doi.org/10.1016/s1074-7613(03)00267-x
Rajaiya J, Nixon JC, Ayers N et al (2006) Induction of immunoglobulin heavy-chain transcription through the transcription factor bright requires TFII-I. Mol Cell Biol 26:4758–4768. https://doi.org/10.1128/MCB.02009-05
Guo M, Price MJ, Patterson DG et al (2018) EZH2 represses the B-cell transcriptional program and regulates antibody-secreting cell metabolism and antibody production. J Immunol 200:1039–1052. https://doi.org/10.4049/jimmunol.1701470
Scherer DC, Brockman JA, Bendall HH et al (1996) Corepression of RelA and c-rel inhibits immunoglobulin kappa gene transcription and rearrangement in precursor B lymphocytes. Immunity 5:563–574
Zelazowski P, Carrasco D, Rosas FR et al (1997) B cells genetically deficient in the c-Rel transactivation domain have selective defects in germline CH transcription and Ig class switching. J Immunol 159:3133–3139
Lin SC, Wortis HH, Stavnezer J (1998) The ability of CD40L, but not lipopolysaccharide, to initiate immunoglobulin switching to immunoglobulin G1 is explained by differential induction of NF-kappaB/Rel proteins. Mol Cell Biol 18:5523–5532. https://doi.org/10.1128/mcb.18.9.5523
Zelazowski P, Shen Y, Snapper CM (2000) NF-kappaB/p50 and NF-kappaB/c-Rel differentially regulate the activity of the 3’alphaE-hsl,2 enhancer in normal murine B cells in an activation-dependent manner. Int Immunol 12:1167–1172. https://doi.org/10.1093/intimm/12.8.1167
Jain A, Ma CA, Lopez-Granados E et al (2004) Specific NEMO mutations impair CD40-mediated c-Rel activation and B-cell terminal differentiation. J Clin Investig 114:1593–1602. https://doi.org/10.1172/JCI21345
Agresti A, Vercelli D (2002) c-Rel is a selective activator of a novel IL-4/CD40 responsive element in the human Ig gamma4 germline promoter. Mol Immunol 38:849–859
Kaku H, Horikawa K, Obata Y et al (2002) NF-kappaB is required for CD38-mediated induction of C(gamma)1 germline transcripts in murine B lymphocytes. Int Immunol 14:1055–1064. https://doi.org/10.1093/intimm/dxf072
Hunter JE, Leslie J, Perkins ND (2016) c-Rel and its many roles in cancer: an old story with new twists. Br J Cancer 114:1–6. https://doi.org/10.1038/bjc.2015.410
Gaspar-Pereira S, Fullard N, Townsend PA et al (2012) The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am J Pathol 180:929–939. https://doi.org/10.1016/j.ajpath.2011.11.007
Wang Y, Rickman BH, Poutahidis T et al (2008) c-Rel is essential for the development of innate and T cell-induced colitis. J Immunol 180:8118–8125. https://doi.org/10.4049/jimmunol.180.12.8118
Eyre S, Hinks A, Flynn E et al (2010) Confirmation of association of the REL locus with rheumatoid arthritis susceptibility in the UK population. Ann Rheum Dis 69:1572–1573. https://doi.org/10.1136/ard.2009.122887
Varadé J, Palomino-Morales R, Ortego-Centeno N et al (2011) Analysis of the REL polymorphism rs13031237 in autoimmune diseases. Ann Rheum Dis 70:711–712. https://doi.org/10.1136/ard.2010.134593
Gilmore TD, Cormier C, Jean-Jacques J, Gapuzan ME (2001) Malignant transformation of primary chicken spleen cells by human transcription factor c-Rel. Oncogene 20:7098–7103. https://doi.org/10.1038/sj.onc.1204898
Chin M, Herscovitch M, Zhang N et al (2009) Overexpression of an activated REL mutant enhances the transformed state of the human B-lymphoma BJAB-cell line and alters its gene expression profile. Oncogene 28:2100–2111. https://doi.org/10.1038/onc.2009.74
Kober-Hasslacher M, Schmidt-Supprian M (2019) The unsolved puzzle of c-Rel in B-cell lymphoma. Cancers (Basel) 11:941. https://doi.org/10.3390/cancers11070941
Barth TF, Bentz M, Leithäuser F et al (2001) Molecular-cytogenetic comparison of mucosa-associated marginal zone B-cell lymphoma and large B-cell lymphoma arising in the gastro-intestinal tract. Genes Chromosomes Cancer 31:316–325. https://doi.org/10.1002/gcc.1150
Barth TFE, Martin-Subero JI, Joos S et al (2003) Gains of 2p involving the REL locus correlate with nuclear c-Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood 101:3681–3686. https://doi.org/10.1182/blood-2002-08-2577
Monti S, Savage KJ, Kutok JL et al (2005) Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 105:1851–1861. https://doi.org/10.1182/blood-2004-07-2947
Lenz G, Wright GW, Emre NCT et al (2008) Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA 105:13520–13525. https://doi.org/10.1073/pnas.0804295105
Curry CV, Ewton AA, Olsen RJ et al (2009) Prognostic impact of C-REL expression in diffuse large B-cell lymphoma. J Hematop 2:20–26. https://doi.org/10.1007/s12308-009-0021-4
Enciso-Mora V, Broderick P, Ma Y et al (2010) A genome-wide association study of Hodgkin’s lymphoma identifies new susceptibility loci at 2p16.1 (REL), 8q24.21 and 10p14 (GATA3). Nat Genet 42:1126–1130. https://doi.org/10.1038/ng.696
Li L, Xu-Monette ZY, Ok CY et al (2015) Prognostic impact of c-Rel nuclear expression and REL amplification and crosstalk between c-Rel and the p53 pathway in diffuse large B-cell lymphoma. Oncotarget 6:23157–23180. https://doi.org/10.18632/oncotarget.4319
Weniger MA, Gesk S, Ehrlich S et al (2007) Gains of REL in primary mediastinal B-cell lymphoma coincide with nuclear accumulation of REL protein. Genes Chromosomes Cancer 46:406–415. https://doi.org/10.1002/gcc.20420
Joos S, Menz CK, Wrobel G et al (2002) Classical Hodgkin lymphoma is characterized by recurrent copy number gains of the short arm of chromosome 2. Blood 99:1381–1387. https://doi.org/10.1182/blood.v99.4.1381
Kalaitzidis D, Davis RE, Rosenwald A et al (2002) The human B-cell lymphoma cell line RC-K8 has multiple genetic alterations that dysregulate the Rel/NF-kappaB signal transduction pathway. Oncogene 21:8759–8768. https://doi.org/10.1038/sj.onc.1206033
Liang M-C, Bardhan S, Porco JA, Gilmore TD (2006) The synthetic epoxyquinoids jesterone dimer and epoxyquinone A monomer induce apoptosis and inhibit REL (human c-Rel) DNA binding in an IκBα-deficient diffuse large B-cell lymphoma cell line. Cancer Lett 241:69–78. https://doi.org/10.1016/j.canlet.2005.10.004
Tian W, Liou H-C (2009) RNAi-mediated c-Rel silencing leads to apoptosis of B-cell tumor cells and suppresses antigenic immune response in vivo. PLoS ONE 4:e5028. https://doi.org/10.1371/journal.pone.0005028
Clodi K, Asgary Z, Zhao S et al (1998) Coexpression of CD40 and CD40 ligand in B-cell lymphoma cells. Br J Haematol 103:270–275. https://doi.org/10.1046/j.1365-2141.1998.01031.x
Pham LV, Tamayo AT, Yoshimura LC et al (2005) Constitutive NF-kappaB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood 106:3940–3947. https://doi.org/10.1182/blood-2005-03-1167
Hunter JE, Butterworth JA, Zhao B et al (2016) The NF-κB subunit c-Rel regulates Bach2 tumour suppressor expression in B-cell lymphoma. Oncogene 35:3476–3484. https://doi.org/10.1038/onc.2015.399
Markopoulos GS, Roupakia E, Tokamani M et al (2018) Roles of NF-κB signaling in the regulation of miRNAs impacting on inflammation in cancer. Biomedicines 6:40. https://doi.org/10.3390/biomedicines6020040
Liu W-H, Kang SG, Huang Z et al (2016) A miR-155-Peli1-c-Rel pathway controls the generation and function of T follicular helper cells. J Exp Med 213:1901–1919. https://doi.org/10.1084/jem.20160204
Chang M, Jin W, Chang J-H et al (2011) The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat Immunol 12:1002–1009. https://doi.org/10.1038/ni.2090
Chang M, Jin W, Sun S-C (2009) Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol 10:1089–1095. https://doi.org/10.1038/ni.1777
Watanabe-Fukunaga R, Brannan CI, Copeland NG et al (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317. https://doi.org/10.1038/356314a0
Fisher GH, Rosenberg FJ, Straus SE et al (1995) Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81:935–946. https://doi.org/10.1016/0092-8674(95)90013-6
O’Reilly LA, Hughes P, Lin A et al (2015) Loss of c-REL but not NF-κB2 prevents autoimmune disease driven by FasL mutation. Cell Death Differ 22:767–778. https://doi.org/10.1038/cdd.2014.168
Campbell IK, Gerondakis S, O’Donnell K, Wicks IP (2000) Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Investig 105:1799–1806. https://doi.org/10.1172/JCI8298
Stathopoulou C, Nikoleri D, Bertsias G (2019) Immunometabolism: an overview and therapeutic prospects in autoimmune diseases. Immunotherapy 11:813–829. https://doi.org/10.2217/imt-2019-0002
Teng X, Li W, Cornaby C, Morel L (2019) Immune cell metabolism in autoimmunity. Clin Exp Immunol 197:181–192. https://doi.org/10.1111/cei.13277
Colamatteo A, Micillo T, Bruzzaniti S et al (2019) Metabolism and autoimmune responses: the microRNA connection. Front Immunol 10:1969. https://doi.org/10.3389/fimmu.2019.01969
Caro-Maldonado A, Wang R, Nichols AG et al (2014) Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 192:3626–3636. https://doi.org/10.4049/jimmunol.1302062
Waters LR, Ahsan FM, Wolf DM et al (2018) Initial B-cell activation induces metabolic reprogramming and mitochondrial remodeling. iScience 5:99–109. https://doi.org/10.1016/j.isci.2018.07.005
de Jesus T, Shukla S, Ramakrishnan P (2018) Too sweet to resist: control of immune cell function by O-GlcNAcylation. Cell Immunol 333:85–92. https://doi.org/10.1016/j.cellimm.2018.05.010
Wu J-L, Chiang M-F, Hsu P-H et al (2017) O-GlcNAcylation is required for B-cell homeostasis and antibody responses. Nat Commun 8:1854. https://doi.org/10.1038/s41467-017-01677-z
Cotugno N, Finocchi A, Cagigi A et al (2015) Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. J Allergy Clin Immunol 135:753–761.e2. https://doi.org/10.1016/j.jaci.2014.07.012
Pozo-Beltrán CF, Suárez-Gutiérrez MA, Yamazaki-Nakashimada MA et al (2019) B subset cells in patients with chronic granulomatous disease in a Mexican population. Allergol Immunopathol (Madr) 47:372–377. https://doi.org/10.1016/j.aller.2019.03.005
Feng Y-Y, Tang M, Suzuki M et al (2019) Essential role of NADPH oxidase-dependent production of reactive oxygen species in maintenance of sustained B-cell receptor signaling and B-cell proliferation. J Immunol 202:2546–2557. https://doi.org/10.4049/jimmunol.1800443
Wheeler ML, Defranco AL (2012) Prolonged production of reactive oxygen species in response to B-cell receptor stimulation promotes B-cell activation and proliferation. J Immunol 189:4405–4416. https://doi.org/10.4049/jimmunol.1201433
Morgan MJ, Liu Z (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115. https://doi.org/10.1038/cr.2010.178
Cieslik KA, Deng W-G, Wu KK (2006) Essential role of C-Rel in nitric-oxide synthase-2 transcriptional activation: time-dependent control by salicylate. Mol Pharmacol 70:2004–2014. https://doi.org/10.1124/mol.106.026054
Shi FD, Flodström M, Kim SH et al (2001) Control of the autoimmune response by type 2 nitric oxide synthase. J Immunol 167:3000–3006. https://doi.org/10.4049/jimmunol.167.5.3000
Beaussant-Cohen S, Jaber F, Massaad MJ et al (2019) Combined immunodeficiency in a patient with c-Rel deficiency. J Allergy Clin Immunol 144:606–608.e4. https://doi.org/10.1016/j.jaci.2019.05.003
Boisson B, Honda Y, Ajiro M et al (2019) Rescue of recurrent deep intronic mutation underlying cell type-dependent quantitative NEMO deficiency. J Clin Investig 129:583–597. https://doi.org/10.1172/JCI124011
Fusco F, Pescatore A, Conte MI et al (2015) EDA-ID and IP, two faces of the same coin: how the same IKBKG/NEMO mutation affecting the NF-κB pathway can cause immunodeficiency and/or inflammation. Int Rev Immunol 34:445–459. https://doi.org/10.3109/08830185.2015.1055331
Beg AA, Sha WC, Bronson RT et al (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376:167–170. https://doi.org/10.1038/376167a0
Jain A, Ma CA, Liu S et al (2001) Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol 2:223–228. https://doi.org/10.1038/85277
Liou HC, Jin Z, Tumang J et al (1999) c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int Immunol 11:361–371. https://doi.org/10.1093/intimm/11.3.361
Milanovic M, Heise N, De Silva NS et al (2017) Differential requirements for the canonical NF-κB transcription factors c-REL and RELA during the generation and activation of mature B cells. Immunol Cell Biol 95:261–271. https://doi.org/10.1038/icb.2016.95
Luu M, Romero R, Bazant J et al (2019) The NF-κB transcription factor c-Rel controls host defense against Citrobacter rodentium. Eur J Immunol 50:292–294. https://doi.org/10.1002/eji.201948314
Acknowledgements
This work was supported by the National Institute of Health, NIH/NIAID grants R21AI144264 and R01AI116730 to P.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basavarajappa, S.C., Ramakrishnan, P. Regulation of B-cell function by NF-kappaB c-Rel in health and disease. Cell. Mol. Life Sci. 77, 3325–3340 (2020). https://doi.org/10.1007/s00018-020-03488-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03488-w