Abstract
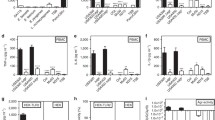
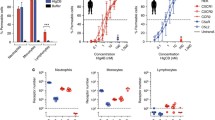
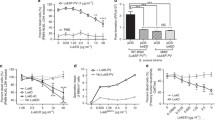
Massive neutrophil infiltration is an early key event in infectious inflammation, accompanied by chemotactic leukotriene (LT)B4 generation. LTB4 biosynthesis is mediated by 5-lipoxygenase (5-LOX), but which pathogenic factors cause 5-LOX activation during bacterial infections is elusive. Here, we reveal staphylococcal exotoxins as 5-LOX activators. Conditioned medium of wild-type Staphylococcus aureus but not of exotoxin-deficient strains induced 5-LOX activation in transfected HEK293 cells. Two different staphylococcal exotoxins mimicked the effects of S. aureus-conditioned medium: (1) the pore-forming toxin α-hemolysin and (2) amphipathic α-helical phenol-soluble modulin (PSM) peptides. Interestingly, in human neutrophils, 5-LOX activation was exclusively evoked by PSMs, which was prevented by the selective FPR2/ALX receptor antagonist WRW4. 5-LOX activation by PSMs was confirmed in vivo as LT formation in infected paws of mice was impaired in response to PSM-deficient S. aureus. Conclusively, exotoxins from S. aureus are potent pathogenic factors that activate 5-LOX and induce LT formation in neutrophils.
Graphic abstract








Similar content being viewed by others
References
Nauseef WM, Borregaard N (2014) Neutrophils at work. Nat Immunol 15:602–611. https://doi.org/10.1038/ni.2921
de Oliveira S, Rosowski EE, Huttenlocher A (2016) Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16:378–391. https://doi.org/10.1038/nri.2016.49
Rådmark O, Werz O, Steinhilber D, Samuelsson B (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta 1851:331–339. https://doi.org/10.1016/j.bbalip.2014.08.012
Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ et al (1990) Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 343:282–284. https://doi.org/10.1038/343282a0
Gerstmeier J, Weinigel C, Rummler S, Radmark O, Werz O et al (2016) Time-resolved in situ assembly of the leukotriene-synthetic 5-lipoxygenase/5-lipoxygenase-activating protein complex in blood leukocytes. FASEB J 30:276–285. https://doi.org/10.1096/fj.15-278010
Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M et al (2018) Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun 9:59. https://doi.org/10.1038/s41467-017-02538-5
Golenkina EA, Galkina SI, Romanova JM, Lazarenko MI, Sud’ina GF (2011) Involvement of red blood cells in the regulation of leukotriene synthesis in polymorphonuclear leucocytes upon interaction with Salmonella typhimurium. APMIS 119:635–642. https://doi.org/10.1111/j.1600-0463.2011.02786.x
Wu HJ, Wang AH, Jennings MP (2008) Discovery of virulence factors of pathogenic bacteria. Curr Opin Chem Biol 12:93–101. https://doi.org/10.1016/j.cbpa.2008.01.023
Peters-Golden M, Henderson WR Jr (2007) Leukotrienes. N Engl J Med 357:1841–1854. https://doi.org/10.1056/nejmra071371
Mancuso P, Nana-Sinkam P, Peters-Golden M (2001) Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun 69:2011–2016. https://doi.org/10.1128/IAI.69.4.2011-2016.2001
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. https://doi.org/10.1038/ni.1863
Surette ME, Palmantier R, Gosselin J, Borgeat P (1993) Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med 178:1347–1355
Bischofberger M, Iacovache I, van der Goot FG (2012) Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12:266–275. https://doi.org/10.1016/j.chom.2012.08.005
DuMont AL, Torres VJ (2014) Cell targeting by the Staphylococcus aureus pore-forming toxins: it’s not just about lipids. Trends Microbiol 22:21–27. https://doi.org/10.1016/j.tim.2013.10.004
Peschel A, Otto M (2013) Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11:667–673. https://doi.org/10.1038/nrmicro3110
Bronner S, Monteil H, Prevost G (2004) Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 28:183–200. https://doi.org/10.1016/j.femsre.2003.09.003
Tuchscherr L, Bischoff M, Lattar SM, Noto Llana M, Pfortner H et al (2015) Sigma factor SigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog 11:e1004870. https://doi.org/10.1371/journal.ppat.1004870
Bremm KD, Konig W, Pfeiffer P, Rauschen I, Theobald K et al (1985) Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun 50:844–851
Suttorp N, Seeger W, Zucker-Reimann J, Roka L, Bhakdi S (1987) Mechanism of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect Immun 55:104–110
Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M et al (2010) Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7:463–473. https://doi.org/10.1016/j.chom.2010.05.012
Gerstmeier J, Weinigel C, Barz D, Werz O, Garscha U (2014) An experimental cell-based model for studying the cell biology and molecular pharmacology of 5-lipoxygenase-activating protein in leukotriene biosynthesis. Biochim Biophys Acta 1840:2961–2969. https://doi.org/10.1016/j.bbagen.2014.05.016
Licona-Limon I, Garay-Canales CA, Munoz-Paleta O, Ortega E (2015) CD13 mediates phagocytosis in human monocytic cells. J Leukoc Biol 98:85–98. https://doi.org/10.1189/jlb.2A0914-458R
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P et al (2015) Proteomics. Tissue-based map of the human proteome. Science 347:1260419. https://doi.org/10.1126/science.1260419
Chart H, Smith HR, La Ragione RM, Woodward MJ (2000) An investigation into the pathogenic properties of Escherichia coli strains BLR, BL21, DH5alpha and EQ1. J Appl Microbiol 89:1048–1058
Gotz F, Schumacher B (1987) Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett 40:285–288. https://doi.org/10.1016/0378-1097(87)90391-0
Los FC, Randis TM, Aroian RV, Ratner AJ (2013) Role of pore-forming t oxins in bacterial infectious diseases. Microbiol Mol Biol Rev 77:173–207. https://doi.org/10.1128/MMBR.00052-12
Porta H, Cancino-Rodezno A, Soberon M, Bravo A (2011) Role of MAPK p38 in the cellular responses to pore-forming toxins. Peptides 32:601–606. https://doi.org/10.1016/j.peptides.2010.06.012
Kramer S, Sellge G, Lorentz A, Krueger D, Schemann M et al (2008) Selective activation of human intestinal mast cells by Escherichia coli hemolysin. J Immunol 181:1438–1445
Werz O, Klemm J, Samuelsson B, Radmark O (2000) 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc Natl Acad Sci USA 97:5261–5266. https://doi.org/10.1073/pnas.050588997
Koeberle SC, Romir J, Fischer S, Koeberle A, Schattel V et al (2011) Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat Chem Biol 8:141–143. https://doi.org/10.1038/nchembio.761
Hammarberg T, Provost P, Persson B, Radmark O (2000) The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J Biol Chem 275:38787–38793. https://doi.org/10.1074/jbc.M006136200
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. https://doi.org/10.1038/nri3024
Brock TG, McNish RW, Bailie MB, PetersGolden M (1997) Rapid import of cytosolic 5-lipoxygenase into the nucleus of neutrophils after in vivo recruitment and in vitro adherence. J Biol Chem 272:8276–8280. https://doi.org/10.1074/jbc.272.13.8276
de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ et al (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695. https://doi.org/10.1084/jem.20031636
Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F et al (2001) The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem 276:21585–21593. https://doi.org/10.1074/jbc.M007769200
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532. https://doi.org/10.1056/NEJM199808203390806
DeLeo FR, Otto M, Kreiswirth BN, Chambers HF (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. https://doi.org/10.1016/S0140-6736(09)61999-1
Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J et al (2018) Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev. https://doi.org/10.1128/CMR.00084-17
Foster TJ (2005) Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958. https://doi.org/10.1038/nrmicro1289
Arya R, Princy SA (2013) An insight into pleiotropic regulators Agr and Sar: molecular probes paving the new way for antivirulent therapy. Future Microbiol 8:1339–1353. https://doi.org/10.2217/fmb.13.92
Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM et al (1994) Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest 94:1815–1822. https://doi.org/10.1172/JCI117530
Dal Peraro M, van der Goot FG (2016) Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol 14:77–92. https://doi.org/10.1038/nrmicro.2015.3
Werz O, Szellas D, Steinhilber D, Radmark O (2002) Arachidonic acid promotes phosphorylation of 5-lipoxygenase at Ser-271 by MAPK-activated protein kinase 2 (MK2). J Biol Chem 277:14793–14800. https://doi.org/10.1074/jbc.M111945200
Wilke GA, Bubeck Wardenburg J (2010) Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci USA 107:13473–13478. https://doi.org/10.1073/pnas.1001815107
Nygaard TK, Pallister KB, Zurek OW, Voyich JM (2013) The impact of alpha-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300. J Leukoc Biol 94:971–979. https://doi.org/10.1189/jlb.0213080
Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY et al (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. https://doi.org/10.1038/nm1656
Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR et al (2010) Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog 6:e1001133. https://doi.org/10.1371/journal.ppat.1001133
Li L, Pian Y, Chen S, Hao H, Zheng Y et al (2016) Phenol-soluble modulin alpha4 mediates Staphylococcus aureus-associated vascular leakage by stimulating heparin-binding protein release from neutrophils. Sci Rep 6:29373. https://doi.org/10.1038/srep29373
Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W et al (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498:371–375. https://doi.org/10.1038/nature12175
Kienle K, Lammermann T (2016) Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 273:76–93. https://doi.org/10.1111/imr.12458
Chiang N, Arita M, Serhan CN (2005) Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids 73:163–177. https://doi.org/10.1016/j.plefa.2005.05.003
Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR et al (2013) Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol 15:1427–1437. https://doi.org/10.1111/cmi.12130
Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D et al (2010) Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. https://doi.org/10.1371/journal.ppat.1000715
Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C et al (2012) The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. https://doi.org/10.1371/journal.ppat.1003016
Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97:77–89
Garscha U, Romp E, Pace S, Rossi A, Temml V et al (2017) Pharmacological profile and efficiency in vivo of diflapolin, the first dual inhibitor of 5-lipoxygenase-activating protein and soluble epoxide hydrolase. Sci Rep 7:9398. https://doi.org/10.1038/s41598-017-09795-w
Nippe N, Varga G, Holzinger D, Loffler B, Medina E et al (2011) Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J Invest Dermatol 131:125–132. https://doi.org/10.1038/jid.2010.282
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (GA2101/2-1, SFB1127 ChemBioSys, and SFB 1278 Polytarget) and SFB-TR156 to C.W., E.R. received a stipend from Landesgraduierten-Akademie Jena. V.A. was supported by the International Leibniz Research School for Microbial and Biomolecular Interactions (ILRS). L.T. and B.L. were supported by the Leibniz Science Campus InfectoOptics SAS-2015-HKI-LWC.
Author information
Authors and Affiliations
Contributions
ER, VA, JF, AS, and CW performed the experiments; ER and UG performed data analysis; ER, OW, and UG designed the study and all authors contributed to the discussion and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Romp, E., Arakandy, V., Fischer, J. et al. Exotoxins from Staphylococcus aureus activate 5-lipoxygenase and induce leukotriene biosynthesis. Cell. Mol. Life Sci. 77, 3841–3858 (2020). https://doi.org/10.1007/s00018-019-03393-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03393-x