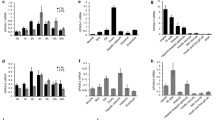
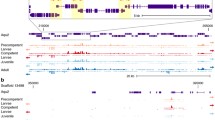
Abstract
The universal nine-amino-acid transactivation domains (9aaTADs) have been identified in numerous transcription activators. Here, we identified the conserved 9aaTAD motif in all nine members of the specificity protein (SP) family. Previously, the Sp1 transcription factor has been defined as a glutamine-rich activator. We showed by amino acid substitutions that the glutamine residues are completely dispensable for 9aaTAD function and are not conserved in the SP family. We described the origin and evolutionary history of 9aaTADs. The 9aaTADs of the ancestral Sp2 gene became inactivated in early chordates. We next discovered that an accumulation of valines in 9aaTADs inactivated their transactivation function and enabled their strict conservation during evolution. Subsequently, in chordates, Sp2 has duplicated and created new paralogs, Sp1, Sp3, and Sp4 (the SP1–4 clade). During chordate evolution, the dormancy of the Sp2 activation domain lasted over 100 million years. The dormant but still intact ancestral Sp2 activation domains allowed diversification of the SP1–4 clade into activators and repressors. By valine substitution in the 9aaTADs, Sp1 and Sp3 regained their original activator function found in ancestral lower metazoan sea sponges. Therefore, the vertebrate SP1–4 clade could include both repressors and activators. Furthermore, we identified secondary 9aaTADs in Sp2 introns present from fish to primates, including humans. In the gibbon genome, introns containing 9aaTADs were used as exons, which turned the Sp2 gene into an activator. Similarly, we identified introns containing 9aaTADs used conditionally as exons in the (SP family-unrelated) transcription factor SREBP1, suggesting that the intron-9aaTAD reservoir is a general phenomenon.








Similar content being viewed by others
References
Teufel DP, Freund SM, Bycroft M, Fersht AR (2007) Four domains of p300 each bind tightly to a sequence spanning both transactivation subdomains of p53. Proc Natl Acad Sci USA 104:7009–7014. https://doi.org/10.1073/pnas.0702010104
Gamper AM, Roeder RG (2008) Multivalent binding of p53 to the STAGA complex mediates coactivator recruitment after UV damage. Mol Cell Biol 28:2517–2527. https://doi.org/10.1128/MCB.01461-07
Feng H, Jenkins LMM, Durell SR et al (2009) Structural basis for p300 Taz2-p53 TAD1 binding and modulation by phosphorylation. Structure 17:202–210. https://doi.org/10.1016/j.str.2008.12.009
Ferreon JC, Lee CW, Arai M et al (2009) Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2. Proc Natl Acad Sci USA 106:6591–6596. https://doi.org/10.1073/pnas.0811023106
Jenkins LMM, Yamaguchi H, Hayashi R et al (2009) Two distinct motifs within the p53 transactivation domain bind to the Taz2 domain of p300 and are differentially affected by phosphorylation. Biochemistry 48:1244–1255. https://doi.org/10.1021/bi801716h
Thakur JK, Arthanari H, Yang F et al (2009) Mediator subunit Gal11p/MED15 is required for fatty acid-dependent gene activation by yeast transcription factor Oaf1p. J Biol Chem 284:4422–4428. https://doi.org/10.1074/jbc.M808263200
Choi Y, Asada S, Uesugi M (2000) Divergent hTAFII31-binding motifs hidden in activation domains. J Biol Chem 275:15912–15916
Uesugi M, Verdine GL (1999) The alpha-helical FXXPhiPhi motif in p53: tAF interaction and discrimination by MDM2. Proc Natl Acad Sci USA 96:14801–14806
Piskacek M (2009) 9aaTADs mimic DNA to interact with a pseudo-DNA binding domain KIX of Med15 (molecular chameleons). Nat Proc. https://doi.org/10.1038/npre.2009.3939.1
Piskacek M (2009) Common transactivation Motif 9aaTAD recruits multiple general co-activators TAF9, MED15, CBP and p300. Nat Proc. https://doi.org/10.1038/npre.2009.3488.2
Di Lello P, Jenkins LMM, Jones TN et al (2006) Structure of the Tfb1/p53 complex: insights into the interaction between the p62/Tfb1 subunit of TFIIH and the activation domain of p53. Mol Cell 22:731–740. https://doi.org/10.1016/j.molcel.2006.05.007
Piskacek S, Gregor M, Nemethova M et al (2007) Nine-amino-acid transactivation domain: establishment and prediction utilities. Genomics 89:756–768. https://doi.org/10.1016/j.ygeno.2007.02.003
Piskacek M, Vasku A, Hajek R, Knight A (2015) Shared structural features of the 9aaTAD family in complex with CBP. Mol BioSyst 11:844–851. https://doi.org/10.1039/c4mb00672k
Piskacek M, Havelka M, Rezacova M, Knight A (2016) The 9aaTAD transactivation domains: from Gal4 to p53. PLoS One 11:e0162842. https://doi.org/10.1371/journal.pone.0162842
Piskacek M (2009) 9aaTAD prediction result (2006). Nat Proc 1:1. https://doi.org/10.1038/npre.2009.3984.1
Sandholzer J, Hoeth M, Piskacek M et al (2007) A novel 9-amino-acid transactivation domain in the C-terminal part of Sox18. Biochem Biophys Res Commun 360:370–374. https://doi.org/10.1016/j.bbrc.2007.06.095
Piskacek M, Havelka M, Rezacova M, Knight A (2017) The 9aaTAD is exclusive activation domain in Gal4. PLoS One 12:e0169261. https://doi.org/10.1371/journal.pone.0169261
Kakidani H, Ptashne M (1988) GAL4 activates gene expression in mammalian cells. Cell 52:161–167
Fields S, Jang SK (1990) Presence of a potent transcription activating sequence in the p53 protein. Science 249:1046–1049
Piskacek M, Havelka M, Jendruchova K, Knight A (2018) Nuclear hormone receptors: ancient 9aaTAD and evolutionally gained NCoA activation pathways. J Steroid Biochem Mol Biol. https://doi.org/10.1016/j.jsbmb.2018.11.008
Triezenberg SJ (1995) Structure and function of transcriptional activation domains. Curr Opin Genet Dev 5:190–196
Ma J, Ptashne M (1987) A new class of yeast transcriptional activators. Cell 51:113–119
Courey AJ, Tjian R (1988) Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55:887–898
Courey AJ, Holtzman DA, Jackson SP, Tjian R (1989) Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell 59:827–836
Tanese N, Pugh BF, Tjian R (1991) Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev 5:2212–2224
Mermod N, O’Neill EA, Kelly TJ, Tjian R (1989) The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell 58:741–753
Stargell LA, Struhl K (1995) The TBP-TFIIA interaction in the response to acidic activators in vivo. Science 269:75–78
Chou S, Struhl K (1997) Transcriptional activation by TFIIB mutants that are severely impaired in interaction with promoter DNA and acidic activation domains. Mol Cell Biol 17:6794–6802
Dorris DR, Struhl K (2000) Artificial recruitment of TFIID, but not RNA polymerase II holoenzyme, activates transcription in mammalian cells. Mol Cell Biol 20:4350–4358
Thoden JB, Ryan LA, Reece RJ, Holden HM (2008) The interaction between an acidic transcriptional activator and its inhibitor. The molecular basis of Gal4p recognition by Gal80p. J Biol Chem 283:30266–30272. https://doi.org/10.1074/jbc.M805200200
Drysdale CM, Dueñas E, Jackson BM et al (1995) The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol Cell Biol 15:1220–1233
Jackson BM, Drysdale CM, Natarajan K, Hinnebusch AG (1996) Identification of seven hydrophobic clusters in GCN4 making redundant contributions to transcriptional activation. Mol Cell Biol 16:5557–5571
Natarajan K, Meyer MR, Jackson BM et al (2001) Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol 21:4347–4368. https://doi.org/10.1128/MCB.21.13.4347-4368.2001
Jedidi I, Zhang F, Qiu H et al (2010) Activator Gcn4 employs multiple segments of Med15/Gal11, including the KIX domain, to recruit mediator to target genes in vivo. J Biol Chem 285:2438–2455. https://doi.org/10.1074/jbc.M109.071589
Krois AS, Ferreon JC, Martinez-Yamout MA et al (2016) Recognition of the disordered p53 transactivation domain by the transcriptional adapter zinc finger domains of CREB-binding protein. Proc Natl Acad Sci USA 113:E1853–1862. https://doi.org/10.1073/pnas.1602487113
Lee CW, Arai M, Martinez-Yamout MA et al (2009) Mapping the interactions of the p53 transactivation domain with the KIX domain of CBP. Biochemistry 48:2115–2124. https://doi.org/10.1021/bi802055v
Denis CM, Chitayat S, Plevin MJ et al (2012) Structural basis of CBP/p300 recruitment in leukemia induction by E2A-PBX1. Blood. https://doi.org/10.1182/blood-2012-02-411397
Wang F, Marshall CB, Li G-Y et al (2009) Synergistic interplay between promoter recognition and CBP/p300 coactivator recruitment by FOXO3a. ACS Chem Biol 4:1017–1027. https://doi.org/10.1021/cb900190u
Radhakrishnan I, Pérez-Alvarado GC, Parker D et al (1997) Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91:741–752
Lee CW, Martinez-Yamout MA, Dyson HJ, Wright PE (2010) Structure of the p53 transactivation domain in complex with the nuclear receptor coactivator binding domain of CREB binding protein. Biochemistry 49:9964–9971. https://doi.org/10.1021/bi1012996
Wojciak JM, Martinez-Yamout MA, Dyson HJ, Wright PE (2009) Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. EMBO J 28:948–958. https://doi.org/10.1038/emboj.2009.30
Gill G, Pascal E, Tseng ZH, Tjian R (1994) A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci USA 91:192–196
Titz B, Thomas S, Rajagopala SV et al (2006) Transcriptional activators in yeast. Nucleic Acids Res 34:955–967. https://doi.org/10.1093/nar/gkj493
Escher D, Bodmer-Glavas M, Barberis A, Schaffner W (2000) Conservation of glutamine-rich transactivation function between yeast and humans. Mol Cell Biol 20:2774–2782
Hahn S (1993) Structure(?) and function of acidic transcription activators. Cell 72:481–483. https://doi.org/10.1016/0092-8674(93)90064-W
Brzovic PS, Heikaus CC, Kisselev L et al (2011) The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol Cell 44:942–953. https://doi.org/10.1016/j.molcel.2011.11.008
Lu Z, Ansari AZ, Lu X et al (2002) A target essential for the activity of a nonacidic yeast transcriptional activator. Proc Natl Acad Sci USA 99:8591–8596. https://doi.org/10.1073/pnas.092263499
Ma J, Ptashne M (1987) Deletion analysis of GAL4 defines two transcriptional activating segments. Cell 48:847–853
Ferreira ME, Hermann S, Prochasson P et al (2005) Mechanism of transcription factor recruitment by acidic activators. J Biol Chem 280:21779–21784. https://doi.org/10.1074/jbc.M502627200
Staller MV, Holehouse AS, Swain-Lenz D et al (2018) A high-throughput mutational scan of an intrinsically disordered acidic transcriptional activation domain. Cell Syst. https://doi.org/10.1016/j.cels.2018.01.015
Zhang H-M, Liu T, Liu C-J et al (2015) AnimalTFDB 2.0: a resource for expression, prediction and functional study of animal transcription factors. Nucleic Acids Res 43:D76–81. https://doi.org/10.1093/nar/gku887
Kolell KJ, Crawford DL (2002) Evolution of Sp transcription factors. Mol Biol Evol 19:216–222
Kaczynski J, Cook T, Urrutia R (2003) Sp1- and Krüppel-like transcription factors. Genome Biol 4:206
Suske G, Bruford E, Philipsen S (2005) Mammalian SP/KLF transcription factors: bring in the family. Genomics 85:551–556. https://doi.org/10.1016/j.ygeno.2005.01.005
Vizcaíno C, Mansilla S, Portugal J (2015) Sp1 transcription factor: a long-standing target in cancer chemotherapy. Pharmacol Ther 152:111–124. https://doi.org/10.1016/j.pharmthera.2015.05.008
Mir R, Sharma A, Pradhan SJ, Galande S (2018) Regulation of transcription factor SP1 by β-catenin destruction complex modulates Wnt response. bioRxiv 308841. https://doi.org/10.1101/308841
Rane MJ, Zhao Y, Cai L (2019) Krϋppel-like factors (KLFs) in renal physiology and disease. EBioMedicine. https://doi.org/10.1016/j.ebiom.2019.01.021
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, New York
Baumgartner U, Hamilton B, Piskacek M et al (1999) Functional analysis of the Zn(2)Cys(6) transcription factors Oaf1p and Pip2p. Different roles in fatty acid induction of beta-oxidation in Saccharomyces cerevisiae. J Biol Chem 274:22208–22216
Leuther KK, Salmeron JM, Johnston SA (1993) Genetic evidence that an activation domain of GAL4 does not require acidity and may form a beta sheet. Cell 72:575–585
Baur F, Nau K, Sadic D et al (2010) Specificity protein 2 (Sp2) is essential for mouse development and autonomous proliferation of mouse embryonic fibroblasts. PLoS One 5:e9587. https://doi.org/10.1371/journal.pone.0009587
Terrados G, Finkernagel F, Stielow B et al (2012) Genome-wide localization and expression profiling establish Sp2 as a sequence-specific transcription factor regulating vitally important genes. Nucleic Acids Res 40:7844–7857. https://doi.org/10.1093/nar/gks544
Völkel S, Stielow B, Finkernagel F et al (2015) Zinc finger independent genome-wide binding of Sp2 potentiates recruitment of histone-fold protein Nf-y distinguishing it from Sp1 and Sp3. PLoS Genet 11:e1005102. https://doi.org/10.1371/journal.pgen.1005102
Ratajewski M, Walczak-Drzewiecka A, Gorzkiewicz M et al (2016) Expression of human gene coding RORγT receptor depends on the Sp2 transcription factor. J Leukoc Biol 100:1213–1223. https://doi.org/10.1189/jlb.6A0515-212RR
Zschemisch N-H, Brüsch I, Hambusch A-S, Bleich A (2016) Transcription factor SP2 enhanced the expression of Cd14 in colitis-susceptible C3H/HeJBir. PLoS One 11:e0155821. https://doi.org/10.1371/journal.pone.0155821
Moorefield KS, Fry SJ, Horowitz JM (2004) Sp2 DNA binding activity and trans-activation are negatively regulated in mammalian cells. J Biol Chem 279:13911–13924. https://doi.org/10.1074/jbc.M313589200
Yin H, Nichols TD, Horowitz JM (2010) Transcription of mouse Sp2 yields alternatively spliced and sub-genomic mRNAs in a tissue- and cell-type-specific fashion. Biochim Biophys Acta 1799:520–531. https://doi.org/10.1016/j.bbagrm.2010.03.002
Phan D, Cheng C-J, Galfione M et al (2004) Identification of Sp2 as a transcriptional repressor of carcinoembryonic antigen-related cell adhesion molecule 1 in tumorigenesis. Cancer Res 64:3072–3078
Yesudhas D, Anwar MA, Panneerselvam S et al (2017) Evaluation of Sox2 binding affinities for distinct DNA patterns using steered molecular dynamics simulation. FEBS Open Bio 7:1750–1767. https://doi.org/10.1002/2211-5463.12316
Kamachi Y, Kondoh H (2013) Sox proteins: regulators of cell fate specification and differentiation. Development 140:4129–4144. https://doi.org/10.1242/dev.091793
Lodato MA, Ng CW, Wamstad JA et al (2013) SOX2 co-occupies distal enhancer elements with distinct POU factors in ESCs and NPCs to specify cell state. PLoS Genet 9:e1003288. https://doi.org/10.1371/journal.pgen.1003288
Ward SV, Samuel CE (2003) The pkr kinase promoter binds both Sp1 and Sp3, but only Sp3 functions as part of the interferon-inducible complex with ISGF-3 proteins. Virology 313:553–566. https://doi.org/10.1016/S0042-6822(03)00347-7
Jaiswal AS, Balusu R, Narayan S (2006) 7,12-Dimethylbenzanthracene-dependent transcriptional regulation of adenomatous polyposis coli (APC) gene expression in normal breast epithelial cells is mediated by GC-box binding protein Sp3. Carcinogenesis 27:252–261. https://doi.org/10.1093/carcin/bgi225
Li L, Davie JR (2010) The role of Sp1 and Sp3 in normal and cancer cell biology. Ann Anat Anatomischer Anz 192:275–283. https://doi.org/10.1016/j.aanat.2010.07.010
Erwin DH, Laflamme M, Tweedt SM et al (2011) The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334:1091–1097. https://doi.org/10.1126/science.1206375
Presnell JS, Schnitzler CE, Browne WE (2015) KLF/SP transcription factor family evolution: expansion, diversification, and innovation in eukaryotes. Genome Biol Evol 7:2289–2309. https://doi.org/10.1093/gbe/evv141
Hackett SJ, Kimball RT, Reddy S et al (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320:1763–1768. https://doi.org/10.1126/science.1157704
Christoffels A, Koh EGL, Chia J-M et al (2004) Fugu genome analysis provides evidence for a whole-genome duplication early during the evolution of ray-finned fishes. Mol Biol Evol 21:1146–1151. https://doi.org/10.1093/molbev/msh114
Conkright MD, Wani MA, Lingrel JB (2001) Lung Krüppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J Biol Chem 276:29299–29306. https://doi.org/10.1074/jbc.M103670200
Geiman DE, Ton-That H, Johnson JM, Yang VW (2000) Transactivation and growth suppression by the gut-enriched Krüppel-like factor (Krüppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction. Nucleic Acids Res 28:1106–1113
Mas C, Lussier-Price M, Soni S et al (2011) Structural and functional characterization of an atypical activation domain in erythroid Kruppel-like factor (EKLF). Proc Natl Acad Sci USA 108:10484–10489. https://doi.org/10.1073/pnas.1017029108
Knights AJ, Yik JJ, Mat Jusoh H et al (2016) Krüppel-like factor 3 (KLF3/BKLF) is required for widespread repression of the inflammatory modulator Galectin-3 (Lgals3). J Biol Chem 291:16048–16058. https://doi.org/10.1074/jbc.M116.715748
Klein RH, Hu W, Kashgari G et al (2017) Characterization of enhancers and the role of the transcription factor KLF7 in regulating corneal epithelial differentiation. J Biol Chem 292:18937–18950. https://doi.org/10.1074/jbc.M117.793117
Das A, Fernandez-Zapico ME, Cao S et al (2006) Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J Biol Chem 281:39105–39113. https://doi.org/10.1074/jbc.M607720200
Zhang H, Zhu X, Chen J et al (2015) Krüppel-like factor 12 is a novel negative regulator of forkhead box O1 expression: a potential role in impaired decidualization. Reprod Biol Endocrinol 13:80. https://doi.org/10.1186/s12958-015-0079-z
Pace CN, Scholtz JM (1998) A helix propensity scale based on experimental studies of peptides and proteins. Biophys J 75:422–427
Pacheco D, Warfield L, Brajcich M et al (2018) Transcription activation domains of the yeast factors Met4 and Ino2: tandem activation domains with properties similar to the yeast Gcn4 activator. Mol Cell Biol. https://doi.org/10.1128/MCB.00038-18
Warfield L, Tuttle LM, Pacheco D et al (2014) A sequence-specific transcription activator motif and powerful synthetic variants that bind Mediator using a fuzzy protein interface. Proc Natl Acad Sci USA 111:E3506–3513. https://doi.org/10.1073/pnas.1412088111
Carbone L, Harris RA, Gnerre S et al (2014) Gibbon genome and the fast karyotype evolution of small apes. Nature 513:195–201. https://doi.org/10.1038/nature13679
Chong S, Dugast-Darzacq C, Liu Z et al (2018) Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. https://doi.org/10.1126/science.aar2555
Acknowledgements
This work was supported by the Ministry of Health of the Czech Republic 15-32935A.
Author information
Authors and Affiliations
Contributions
MP, MH and KJ performed the experiments. MP conceived the project. MP, AK and LPK wrote the manuscript. All authors have contributed critical intellectual content and have approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piskacek, M., Havelka, M., Jendruchova, K. et al. The evolution of the 9aaTAD domain in Sp2 proteins: inactivation with valines and intron reservoirs. Cell. Mol. Life Sci. 77, 1793–1810 (2020). https://doi.org/10.1007/s00018-019-03251-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03251-w