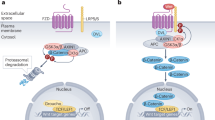
Abstract
Wnt ligands signal through canonical or non-canonical signaling pathways. Although both routes share common elements, such as the Fz2 receptor, they differ in the co-receptor and in many of the final responses; for instance, whereas canonical Wnts increase β-catenin stability, non-canonical ligands downregulate it. However, both types of ligands stimulate tumor cell invasion. We show here that both the canonical Wnt3a and the non-canonical Wnt5a stimulate Fz2 tyrosine phosphorylation, Fyn binding to Fz2, Fyn activation and Fyn-dependent Stat3 phosphorylation. Wnt3a and Wnt5a require Src for Fz2 tyrosine phosphorylation; Src binds to canonical and non-canonical co-receptors (LRP5/6 and Ror2, respectively) and is activated by Wnt3a and Wnt5a. This Fz2/Fyn/Stat3 branch is incompatible with the classical Fz2/Dvl2 pathway as shown by experiments of over-expression or depletion. Fyn is necessary for transcription of genes associated with invasiveness, such as Snail1, and for activation of cell invasion by both Wnt ligands. Our results extend the knowledge about canonical Wnt pathways, demonstrating additional roles for Fyn in this pathway and describing how this protein kinase is activated by both canonical and non-canonical Wnts.








Similar content being viewed by others
References
MacDonald BT, He X (2013) Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol 4:a007880. https://doi.org/10.1101/cshperspect.a007880
Kikuchi A, Yamamoto H, Sato A, Matsumoto S (2012) Wnt5a: its signalling, function and implications in disease. Acta Physiol 204:17–33. https://doi.org/10.1111/j.1748-1716.2011.02294.x
Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R et al (2010) Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated receptors. Genes Dev 24:2517–2530. https://doi.org/10.1101/gad.1957710
Green J, Nusse R, van Amerongen R (2014) The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol 6:a009175. https://doi.org/10.1101/cshperspect.a009175
Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ et al (2004) Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem 279:13011–13017. https://doi.org/10.1074/jbc.M304682200
Bryja V, Schulte G, Rawal N, Grahn A, Arenas E (2007) Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci 120:586–595. https://doi.org/10.1242/jcs.03368
Vinyoles M, Del Valle-Pérez B, Curto J, Padilla M, Villarroel A, Yang J et al (2017) Activation of CK1ε by PP2A/PR61ε is required for the initiation of Wnt signaling. Oncogene 36:429–438. https://doi.org/10.1038/onc.2016.209
Curto J, Del Valle-Pérez B, Villarroel A, Fuertes G, Vinyoles M, Peña R et al (2018) CK1ε and p120-catenin control Ror2 function in noncanonical Wnt signaling. Mol Oncol 12:611–629. https://doi.org/10.1002/1878-0261.12184
Casagolda D, Del Valle-Pérez B, Valls G, Lugilde E, Vinyoles M, Casado-Vela J et al (2010) A p120-catenin-CK1epsilon complex regulates Wnt signaling. J Cell Sci 123:2621–2631. https://doi.org/10.1242/jcs.067512
Duñach M, Del Valle-Pérez B, García de Herreros A (2017) p120-catenin in canonical Wnt signalling. Crit Rev Biochem Mol Biol 52:327–339. https://doi.org/10.1038/nrc1752
Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A (2010) Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO 29:41–54. https://doi.org/10.1038/emboj.2009.322
Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F (2008) Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133:340–353. https://doi.org/10.1016/j.cell.2008.01.052
Topol L, Jiang X, Choi H, Garret-Beal L, Carolan PJ, Yang Y (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol 162:899–908. https://doi.org/10.1083/jcb.200303158
Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath GA (2014) A noncanonical Frizzled2 pathway regulates epithelial–mesenchymal transition and metastasis. Cell 159:844–856. https://doi.org/10.1016/j.cell.2014.10.032
Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP et al (1999) Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem 274:19465–19472. https://doi.org/10.1074/jbc.274.27.19465
Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z et al (2004) A mechanism for Wnt coreceptor activation. Mol Cell 13:149–156. https://doi.org/10.1016/S1097-2765(03)00484-2
Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A et al (2003) p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol 23:2287–2297. https://doi.org/10.1128/MCB.23.7.2287-2297.2003
Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W (2004) Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev 18:2225–2230. https://doi.org/10.1101/gad.317604
Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, Heath JK (2008) The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS One 3:e1873. https://doi.org/10.1371/journal.pone.0001873
Chen Q, Su Y, Wesslowski J, Hagemann AI, Ramialison M, Wittbrodt J et al (2014) Tyrosine phosphorylation of LRP6 by Src and Fer inhibits Wnt/β-catenin signalling. EMBO Rep 15:1254–1267. https://doi.org/10.15252/embr.201439644
Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil J (2003) Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci USA 100:13298–13302. https://doi.org/10.1073/pnas.2336149100
Gon H, Fumoto K, Ku Y, Matsumoto S, Kikuchi A (2013) Wnt5a signaling promotes apical and basolateral polarization of single epitelial cells. Mol Biol Cell 24:3764–3774. https://doi.org/10.1091/mbc.E13-07-0357
Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D et al (2003) Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell 12:1251–1260. https://doi.org/10.1016/S1097-2765(03)00427-1
Roura S, Miravet S, Piedra J, García de Herreros A, Duñach M (1999) Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 274:36734–36740. https://doi.org/10.1074/jbc.274.51.36734
Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J et al (2007) Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Mol Cell Biol 27:3804–3816. https://doi.org/10.1128/MCB.01590-06
Kani S, Oishi I, Yamamoto H, Yoda A, Suzuki H, Nomachi A et al (2004) The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase epsilon. J Biol Chem 279:50102–50109. https://doi.org/10.1074/jbc.M409039200
Gerlach JP, Jordens I, Tauriello DVF, Land-Kuper I, Bugter J, Noordstra I et al (2018) TMEM59 potientiates Wnt signaling by promoting signalosome formation. Proc Natl Acad Sci USA 115:3996–4005. https://doi.org/10.1073/pnas.1721321115
Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J et al (1995) Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269:81–83. https://doi.org/10.1126/science.7541555
Cao X, Tay A, Guy GR, Tan YH (1996) Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol 16:1595–1603. https://doi.org/10.1128/MCB.16.4.1595
Imada S, Murata Y, Kotani T, Hatano M, Sun C, Konno T et al (2016) Role of Src family kinases in regulation of intestinal epithelial homeostasis. Mol Cell Biol 36:2811–2823. https://doi.org/10.1128/MCB.00311-16
Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S (2005) Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 280:41342–41351. https://doi.org/10.1074/jbc.M502168200
Yokoyama N, Malbon CC (2009) Dishevelled-2 docks and activates Src in a Wnt-dependent manner. J Cell Sci 122:4439–4451. https://doi.org/10.1242/jcs.051847
Fragoso MA, Patel AK, Nakamura RE, Yi H, Surapaneni K, Hackam AS (2012) The Wnt/β-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells. PLoS One 7:e46892. https://doi.org/10.1371/journal.pone.0046892
Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D et al (2010) Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol 30:3610–3619. https://doi.org/10.1128/MCB.00177-10
Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A et al (2010) Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 29:2036–2046. https://doi.org/10.1038/onc.2009.496
Thiele S, Göbel A, Rachner TD, Fuessel S, Froehner M, Muders MH et al (2015) WNT5A has anti-prostate cancer effects in vitro and reduces tumor growth in the skeleton in vivo. J Bone Miner Res 30:471–480. https://doi.org/10.1002/jbmr.2362
Ren D, Dai Y, Yang Q, Zhang X, Guo W, Ye L et al (2019) Wnt5a induces and maintains prostate cancer cells dormancy in bone. J Exp Med 216:428–449. https://doi.org/10.1084/jem.20180661
Alba-Castellón L, Olivera-Salguero R, Mestre-Farrera A, Peña R, Herrera M, Bonilla F et al (2016) Snail1-dependent activation of cancer-associated fibroblast controls Epithelial tumor cell invasion and metastasis. Cancer Res 76:6205–6217. https://doi.org/10.1158/0008-5472.CAN-16-0176
Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L et al (2003) Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci USA 100:3422–3427. https://doi.org/10.1073/pnas.0130233100
Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T et al (2006) The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108:965–973. https://doi.org/10.1182/blood-2005-12-5046
Baarsma HA, Skronska-Wasek W, Mutze K, Ciolek F, Wagner DE, John-Schuster G et al (2017) Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med 214:143–163. https://doi.org/10.1084/jem.20160675
Valls G, Codina M, Miller RK, Del Valle-Pérez B, Vinyoles M, Caelles C et al (2012) Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin. J Cell Sci 125:5288–5301. https://doi.org/10.1242/jcs.101030
Solanas G, Porta-de-la-Riva M, Agustí C, Casagolda D, Sánchez-Aguilera F, Larriba MJ et al (2008) E-cadherin controls beta-catenin and NF-kappaB transcriptional activity in mesenchymal gene expression. J Cell Sci 121:2224–2234. https://doi.org/10.1242/jcs.021667
Acknowledgements
We thank Drs. A. Muñoz, Y. Minami, J.M. González-Sancho, A. Kikuchi, and A.C. Carrera for kindly providing reagents. This research was funded by Grants from the Ministerio de Economía y Competitividad (MINECO) co-funded by Fondo Europeo de Desarrollo Regional-FEDER-UE to MD (BFU2015-65153-R) and AGH (SAF2016-76461-R). We also appreciate support from ICREA Academia and Instituto de Salud Carlos III (PIE15/00008). GF was recipient of a predoctoral fellowship from FPI (MINECO).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Villarroel, A., del Valle-Pérez, B., Fuertes, G. et al. Src and Fyn define a new signaling cascade activated by canonical and non-canonical Wnt ligands and required for gene transcription and cell invasion. Cell. Mol. Life Sci. 77, 919–935 (2020). https://doi.org/10.1007/s00018-019-03221-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03221-2