Abstract
The gastrointestinal tract is the site of nutrient digestion and absorption and is also colonized by diverse, highly mutualistic microbes. The intestinal microbiota has diverse effects on the development and function of the gut-specific immune system, and provides some protection from infectious pathogens. However, interactions between intestinal immunity and microorganisms are very complex, and recent studies have revealed that this intimate crosstalk may depend on the production and sensing abilities of multiple bioactive small molecule metabolites originating from direct produced by the gut microbiota or by the metabolism of dietary components. Here, we review the interplay between the host immune system and the microbiota, how commensal bacteria regulate the production of metabolites, and how these microbiota-derived products influence the function of several major innate and adaptive immune cells involved in modulating host immune homeostasis.
Similar content being viewed by others
Introduction
Commensal bacteria, with an estimated > 1013 cells in the mammalian gastrointestinal tract, are key players in the maintenance of stable gut physiology and organismal homeostasis. The gut microbiota is not only essential for the digestion, absorption, and storage of food substrates, but also for host immune system development, especially with regard to regulating the homeostasis of the gut immune system [1]. Moreover, balanced immune homeostasis may promote and limit the growth of beneficial and harmful microbes, respectively [2].
The mammalian immune system has coevolved with the complex community of indigenous microbiota that constitutively colonize all barrier surfaces [3]. Indeed, as opposed to existing in isolation, microorganisms regulate multiple aspects of host functions, including metabolism and immune system development [4]. The interaction between the microbiota and host is most prominent on the mucosal surface, which provides an interface between the largely microbe-free host, the microbiota, and the environment. Multiple mucosal defenses maintain compartmentalization of the gut microbiota in the intestinal lumen, such as a specialized mucosal layer, antimicrobial peptides, secreted immunoglobulins and a diverse array of mucosal lymphocytes [5]. Specific changes in gut microbiota composition may activate the mucosal immune system, resulting in chronic inflammation and the development of mucosal injury [6].
Overall, modulation of host metabolism and immunity by gut microorganisms depends mainly on the exchange of small molecules, termed metabolites, between the gut lumen and the epithelial surface. The gut microbiota synthesizes, modulates, and degrades a variety of metabolites, thereby acting as a functional complement to host metabolism, especially for dietary components that the host cannot metabolize [7]. Most metabolites originate in one of two ways: (1) diet-dependent microbial products or (2) diet-independent microbial products. Diet-dependent microbial products are directly linked to diet or digestion; examples of such products include but are not limited to short-chain fatty acids (SCFAs), secondary bile acids, indole and indole derivatives. Diet-independent microbial products are synthesized de novo by gut microbes; examples of these products include lipopolysaccharides and peptidoglycans [8]. More than 50% of the metabolites in feces and urine are derived from or modified by the gut microbiota [9].
Recent studies have identified a critical role for the metabolites derived from commensal bacteria in modulating the homeostasis and function of innate and adaptive immune cells through indirect and direct mechanisms [10,11,12]. Small molecules diffuse directly through the hard mucosal layer to trigger epithelial signals via metabolite-specific receptors, thereby activating signal transduction pathways and transcriptional programs that control the differentiation, proliferation, maturation and effector functions of many cells. These sensing receptors are expressed in different combinations in mucosal cell subsets, such as intestinal epithelial cells (IECs), macrophages (MΦ), dendritic cells (DCs), T cells, and innate lymphoid cells (ILCs), and thus have a critical role in host-microbiota mutualistic interactions [13]. In addition, some metabolites may serve as signaling molecules for quorum sensing and interbacterial communication.
Although it is known that the gut microbiota modulates host biology in numerous ways, many known metabolites have not yet been functionally characterized, and the molecular mechanism involved in these reciprocal interactions is still poorly understood. Here, we summarize the current knowledge of the interaction between the gut microbiota and intestinal immunity, describe the production of several immunomodulatory metabolites, and highlight how these metabolites affect and regulate immune cells to maintain gut health.
The gut microbiota and immune system development
The immune system is responsible for detecting and repairing physiological disorders through both immune responses and critical regulatory processes [14, 15]. However, relative to other organs, the intestinal immune system faces unique challenges resulting from continuous exposure to significant microbial loads. The gut microbiota does not simply evade the immune system to persist in the gastrointestinal tract, and recent research has shown that these microorganisms contribute to the development, maturation, and regulation of the immune system [16, 17]. Due to the abundance and diversity of the gut microbiota, it is important to identify individual species within these communities and their associated effects on the immune system, especially for uncultured or low-abundance microorganisms. Germ-free animals and fecal microbiota transplantation (FMT) are techniques that have been widely used to study the contribution of microbiota (individual species or healthy communities) to the host immune system.
The immune system under a germ-free state
Germ-free animals have a severely immunodeficient immune system and thus, a higher susceptibility to infection, and this immunodeficiency is especially pronounced in the gut. For instance, germ-free animals exhibit low immunoglobulin A (IgA) concentrations in the intestinal lumen and underdeveloped of gut-associated lymphoid tissues, including small and few Peyer’s patches (PP) and mesenteric lymph nodes (MLNs) [16, 18, 19]. In addition, convincing evidence derived from studies on germ-free animals suggests that commensal intestinal bacteria are essential for the development and function of lymphocyte subsets. Bifidobacterium adolescentis and segmented filamentous bacteria (SFBs) have been identified as potent inducers of IL-17-producing T helper (Th) 17 cells [20, 21]. Mice lacking SFBs among gut microbiota have fewer Th17 cells than mice with abundant SFBs, and normal development of Th17 cells can be restored by SFB supplementation [22]. Similarly, monoassociations of germ-free mice with specific Clostridia and Bacteroides species can promote the differentiation of regulatory T cells (Tregs) and repress Treg reduction in the lamina propria (LP) of germ-free mice [23, 24]. Intestinal microbial species were found to be targeted and coated by secretory IgA and alteration of the IgA response leads to reductions in overall microbial diversity. Secretory IgA in the gut enhances the translocation of microbes into lymphoid tissues to facilitate antigen presentation [25,26,27]. The development and maturation of IgA-producing plasma cells are deficient in the gastrointestinal tract of germ-free mice, and IgA levels are thus low [28,29,30]. The intestinal mucus layer, which acts as a barrier to protect the epithelium from irritation by the luminal contents, is thinner in germ-free animals than in conventional animals [31, 32], but its thickness can be recovered after colonization with Lactobacillus plantarum [33].
Immune system alteration during dysbiosis and balance
Antibiotics are one of the greatest achievements in the history of medicine, but their long-term application leads to disruption of intestinal microbial communities [34]. Moreover, many antibiotic compounds increase the host’s susceptibility to several pathogens such as Salmonella species and vancomycin-resistant Enterococcus spp. [35].
In general, profound evidence demonstrates the impact of antibiotic treatment on the intestinal ecosystem and immune system. Mice treated with vancomycin or colistin from birth display a reduced number of isolated lymphoid follicles in the small and large intestine [36]. In addition, mice treated with a mixture of antibiotics consisting of vancomycin, neomycin, and metronidazole exhibit reduced antimicrobial peptide expression [37]. After microbiota depletion by broad-spectrum antibiotic treatment, the Treg cell populations in MLNs, PPs and the colon LP are also reduced, although recolonization by conventional microbiota strains or select species of bacteria can restore these cell populations [38, 39]. Similarly, compared with nontreated mice, antibiotic-treated mice have fewer mucosal Th17 cells and ILCs, which play roles in resistance to extracellular microbiota and pathogenesis by secreting IL-17 and IL-22 [38, 40]. Overall, the impacts of antimicrobial treatment on the immune system are mainly due to changes in microbial community composition.
FMT refers to the entire transfer of fecal microbiota from a healthy donor to a recipient’s intestinal tract to normalize the composition and functionality of the intestinal microbiota. FMT can be applied to normalize the composition of the gut microbiota and increase the proportion and diversity of beneficial bacteria, thereby reducing gut inflammation. FMT also provides the signals necessary for epithelial regeneration, induces the production of mucins and antimicrobial peptides, and reduces bowel permeability to maintain epithelial barrier integrity [41, 42]. Furthermore, FMT stimulates the intestinal adaptive immune response through the Toll-like receptor (TLR) pathway to promote the synthesis of immunoglobulins (e.g., IgA, IgG, and IgM), thereby protecting the intestinal mucosa [43].
Ira Ekmekciu et al., showed treating conventional C57BL/6j mice with a broad-spectrum antibiotic for 8 weeks profoundly changed the immune cell repertoire, and the CD4+ cell production of some cytokines (IFN-γ, IL-17, IL-22 and IL-10) declined. FMT can reconstitute the intestinal microbiota and restore the numbers of CD4+, CD8+, and B220+ cells in the small intestine and colonic CD4+ cells as early as 7 days after transfer [44]. The same author conducted a similar study to investigate the abilities of the FMT of Escherichia coli, Lactobacillus johnson and complex murine microbiota to restore immune function in mice that were immunosuppressed by antibiotic-induced microbiota depletion. Compared with the administration of individual commensal bacteria, FMT appears to be more effective at restoring the decreased numbers of cytokine-producing CD4+ T cells in the mucosal and systemic compartments and maintaining immune functions [45]. Similar experiments in a piglet model also indicated that FMT can modulate the metabolic function of the gut microbiota and enhance the microbiota-derived catabolite tryptophan, increasing the production of IL-22 to maintain the intestinal barrier of newborn piglets [41].
Production of microbiota-derived metabolites
Typically, the relationship between the host and the gut microbiota is highly mutualistic. In general, the host provides food and shelter for bacteria, which in turn helps the host digest complex foods and synthesize essential nutrients (e.g., vitamins B and K) [46]. The host may in fact rely on the gut microbiota for digestion and metabolism. Through the fermentation of undigested dietary components that reach the large intestine, as well as endogenous compounds generated by the microbial communities, the gut microbiota produces an extraordinarily wide range of metabolites [4].
As mentioned above, most metabolites originate from diet-dependent and diet-independent sources [8]. The latter metabolites (e.g., ATP and polysaccharide A) are synthesized de novo by gut microbes. Diet-dependent microbial products can be broadly classified into two categories: (1) directly generated digestion or fermentation of dietary components by the gut microbiota and (2) products of host metabolism, that are biochemically modified by the gut microbiota. Using mass spectrometry, Matsumoto et al., found 179 metabolites in the colonic lumens of mice, but 48 were not present in the food consumed by these mice; among these metabolites, 35 had differential concentrations between the colons of germ-free mice and conventional mice [47]. In fact, nearly 10% of metabolites in the blood as well as more than 50% of those in the feces and urine are derived from or modified by the gut microbiota [9, 48]. In the following sections, we describe the production of the most prominent bacteria-derived diet-dependent metabolites.
Microbial modification of dietary component derived metabolites
Short-chain fatty acids
SCFAs are volatile fatty acids with a 1–6 carbon atoms backbones and are produced in the large intestine through the bacterial fermentation of undigested polysaccharides. Members of Firmicutes are the main producers of butyrate, whereas Bacteroidetes produces most acetates and propionates [85, 86]. Several bacterial species have been reported to be capable of producing SCFAs (Table 1).
Acetate is the most abundant SCFA in the colon, accounting for more than half of the total SCFAs detected in feces [87]. Production of acetate by gut bacteria involves two major metabolic pathways. The majority of acetate is the byproduct of undigested polysaccharide fermentation by most enteric bacteria. In addition, nearly one-third of acetate is derived from acetogenic bacteria, such as Blautia hydrogenotrophica, which can use H2 and CO2 or formic acid to synthesize acetate via the Wood-Ljungdahl pathway [49, 50].
Three main pathways participate in the formation of propionate by gut bacteria: the succinate pathway, the acrylate pathway and the propanediol pathway. Most propionate is formed by Bacteroidetes while utilizing the succinate pathway as a substrate. The acrylate pathway is used to convert lactate to propionate through several enzymatic reactions, and this pathway appears to be limited to a few members of the families Veillonellaceae (e.g., Megasphaera spp.) and Lachnospiraceae (e.g., Coprococcus catus and Clostridium lactatifermentans) [85]. The propanediol pathway is involved in the conversion of deoxy-sugars (rhamnose and fucose) to propionate and can be found in Proteobacterium Salmonella enterica serovar Typhimurium and Lachnospiraceae bacteria [88, 89].
The production of butyrate originates from two molecules of acetyl-CoA and two different pathways for the liberation of butyrate from butyryl-CoA: the acetate CoA-transferase pathway and the butyrate kinase pathway [51, 56]. The latter converts butyryl-CoA into butyrate using phosphotransbutyrylase and butyrate kinase. However, this pathway is not common and is mainly limited to some members of Coprococcus (e.g., Coprococcus eutactus and Coprococcus comes) [85]. The butyryl-CoA:acetate CoA-transferase route is utilized in most known butyrate-producing gut strains (e.g., Eubacterium rectangle, Roseburia spp. Coprococcus cactus, Faecalibacterium prausnitzii, Anaerostipes spp. and Eubacterium hallii) [89].
The production of SCFAs by bacteria is not unique. Depending on the growth substrate, the fermentation products produced by bacteria can change. For instance, butyrate can be found when Roseburia inulinivorans is cultured with glucose, whereas propionate, propanol and butyrate are produced when cultured with fructose [89]. Although carbohydrates are the main source of SCFAs, branched-chain amino acids, known as branched-chain SCFAs (BSCFAs), can be converted into isobutyrate, isovalerate, and 2-methyl butyrate, but they contribute very little (5%) to total SCFA production [90].
Tryptophan metabolites
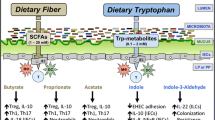
The gastrointestinal tract harbors billions of gut microorganisms that contribute to the first-pass metabolism of dietary components. Tryptophan is utilized to synthesize proteins, yet intestinal bacteria can directly utilize this amino acid to produce many immunologically important metabolites [65, 91], with indole, indolic acid derivatives and tryptamines being the main microbial tryptophan metabolites in the gut [92] (Fig. 1). In addition, indole and indolic acid derivatives can be further metabolized into other final products, such as the conversion of indole-3-acetate to skatole or indole-3-aldehyde, indole to indicant, and indole acrylic acid to indole propionic acid [93] (Fig. 1).
Tryptophan metabolic pathways in the host and microbiota. Among microbial metabolites, indole and indolic acid derivatives are the predominant Trp microbial metabolites in the gut, and the intestinal microbiota produce different metabolites based on which catalytic enzymes the bacteria produce. The kyn and serotonin pathways are the primary routes of host Trp metabolism. Trp tryptophan, TpH trytophan hydroxylase, 5-HTP 5-hydroxy tryptophan, 5-HT serotonin, IDO1 indoleamine 2,3-dioxygenase, TMO tryptophan decarboxylase, TrD tryptophan decarboxylase, ArAT aromatic amino acid aminotransferases, ILDHase indole-3-lactic acid dehydrogenase, TNA tryptophanase
Many bacterial species can convert tryptophan into indole and indole derivatives (Table 1). As one of the major bacterial tryptophan metabolites, indole has been recognized as an important interspecies and interkingdom signaling molecule for biofilm formation as well as for regulating bacterial motility and resisting the invasion of nonindole-producing species, such as Salmonella enterica and Pseudomonas aeruginosa johnsonii) [94]. Through the activity of the enzyme tryptophanase, a variety of bacteria, including Achromobacter liquefaciens, Paracolobactrum coliforms, Bacteroides spp. and E. coli, can convert tryptophan into indole, and a variety of tryptophan microbial metabolites are indolic acid derivatives, such as indole-3-aldehyde, indole lactic acid, indole-3-acetic acid and indole acrylic acid [95]. Via aromatic amino acid aminotransferase, Lactobacillus reuteri and Lactobacillus johnsonii convert tryptophan to indole-3-aldehyde which contributes to the maintenance of intestinal homeostasis by preventing the colonization of pathogenic microorganisms (such as Candida albicans) and weakening inflammatory disorders affecting the intestinal tract [65]. Skatole is not directly synthesized from tryptophan but is decarboxylated by indole-3-acetic acid [92] (Fig. 1). Some species belonging to Lactobacillus, Bacteroides and Clostridium can convert indole-3-acetic acid into skatole [67, 96], which can affect the growth and reproduction of several bacteria, such as Salmonella, Shigella and Escherichia [92]. Tryptophan metabolism by gut bacteria involves multiple catalytic reactions; various bacteria may possess the same enzymes to produce the same metabolites, or a bacterium may produce different tryptophan metabolites. Bacteroides spp. and E. coli are capable of converting tryptophan to indole and tryptamine [95], whereas Clostridia can catabolize tryptophan to indole pyruvic acid, which is then converted to indole-3-acetic acid [93].
In addition to microbial-derived tryptophan metabolites, cells in the liver and extrahepatic tissues can generate tryptophan metabolites such as kynurenine (kyn) and serotonin (5-HT) [97] (Fig. 1). The kyn pathway is the primary route by which the host metabolizes tryptophan through indoleamine 2,3-dioxygenase (IDO1) in the intestine. IDO1 carries out the catabolic conversion of tryptophan to kyn, and kyn can then be used to generate kynurenic acid (KA) or xanthurenic acid (XA) via different routes [98]. Approximately 95% of the tryptophan ingested is broken down via the kyn pathway, ~ 1–2% is used for protein synthesis, and ~ 1–2% is used for the 5-HT pathway [99].
Unlike the relatively simple background of host endogenous tryptophan metabolism, the intestinal environment is extremely complex with regard to bacterial tryptophan metabolism, and many strains that possess catalytic enzymes capable of metabolizing tryptophan are still unknown. Therefore, substantially more research on tryptophan metabolizing bacteria is needed.
Microbial modification of host-derived metabolites
Secondary bile acids
Bacterial metabolites are sourced from not only dietary components but also from substrates secreted into the gut lumen via host metabolism[100]. Primary bile acids, such as cholic acid (CA; 3α, 7α, 12α-trihydroxy-5β-cholan-24-oic acid) and chenodeoxycholic acid (CDCA; 3α, 7α-dihydroxy-5β-cholan-24-oic acid), are derived from cholesterol catabolism in the liver and are further secreted by the gall bladder into the intestine after conjugation to glycine (in humans) or taurine (in mice) [17] (Fig. 2). Nearly 95% of bile acids can be reabsorbed in their conjugated form in the terminal ileum through active transport via apical sodium-dependent bile acid transporters (ASBT) [101], and bile acids that escape active transport in the distal ileum become substrates of colonic bacteria for biotransformation reactions. Several bacteria that metabolize bile acids are shown in Table 1. Biotransformation of bile salts involves multiple steps and typically begins with the deconjugation of taurine or glycine by bile salt hydrolase (BSH) [102]. These deconjugated bile acids are further metabolized via 7-dehydroxylation into secondary bile acids by the microbiota. Lithocholic acid (LCA) and deoxycholic acid (DCA) are the main secondary bile acids resulting from unabsorbed chenodeoxycholic acid (CDCA) and CA, respectively [102,103,104]. The complicated 7-dehydroxylation process involves several reactions carried out by microbiota strains harboring bile acid-inducible (bai) genes [105]. Bacteria capable of producing secondary bile acids belong to the genera Bacteroides, Lactobacillus, Bifidobacterium, Clostridium (clusters XIVa and XI) and Eubacterium [76, 77, 102].
Biosynthesis of bile acids and microbial modification of bile acid metabolism. Cholesterol is converted into two primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), in the liver and further conjugated to glycine or taurine. The bile salts that escape active transport in the distal ileum become substrates for biotransformation reactions by intestinal bacteria. In the intestine, especially in the colon, these conjugated species are first deconjugated to CA or CDCA, and then dehydrogenated and dehydroxylated by gut bacteria into secondary bile acids such as DCA and LCA
Oxo- (or keto) bile acids are other products of bile acid microbial biotransformation formed via gut microbial oxidation by hydroxysteroid dehydrogenases (HSDHs), which have been identified in Proteobacteria, Actinobacteria, and Firmicutes [106, 107]. As these oxidation reactions are reversible and epimerization may occur during the process, some iso-bile acids are found in the serum and urine, particularly in feces and colon digesta [108]. Bacteria capable of producing iso-bile acids include Eubacterium lentum, Clostridium perfringens and Ruminococcus ganvus [78,79,80]. Overall, microbial bile acid metabolism plays an important role in modulating the extent of bile acid recycling by the host.
Metabolic cross-feeding
The intestine provides a nutrient-rich environment for its commensal microorganisms, which compete intensely with each other for nutrition and space but also cooperate in metabolism [109, 110]. Indeed, the metabolite signals derived from microbes function in not only the host but also in other microbiota strains, and the interactions between such communities may significantly effect microbial ecology and physiology. Bacteria can exchange metabolites through the extracellular environment and directly exchange nutrients through cell–cell connections [111]. Microbiota strains are found in a variety of communities in the gastrointestinal tract, and such communities are thought to be largely shaped by interspecies competition for available nutrients or syntrophic interactions (such as beneficial metabolic exchange); the latter can also be called metabolic cross-feeding [112]. Bacterial cross-feeding refers to the utilization of products from the metabolism of one bacteria by another.
Types of bacterial cross-feeding include metabolic cross-feeding and substrate cross-feeding; the former denotes the utilization of metabolic end products produced by a microbiota strain, whereas the latter denotes the utilization of intermediate products formed by a microorganism [113, 114]. Lactate, a common end product of bacterial fermentation produced by Lactobacillus and Bifidobacterium, can be used by other bacteria via metabolite cross-feeding to produce SCFAs. For instance, E. hallii cannot grow in pure culture in the presence of starch, but B. adolescentis grows well in the presence of lactate. When these two strains are co-cultured, the concentration of lactate significantly decreases, while that of butyrate increases. Thus, E. hallii can produce butyric acid using the lactose produced by B. adolescentis. A similar pathway can be found in the acetate producer Desulfovibrio piger and some Firmicutes bacteria [115, 116].
Substrate cross-feeding is more similar to the cooperation between multiple bacteria, such as Roseburia spp., which cannot utilize lactate for growth, but grows well by utilizing breakdown products, namely, fructose by Bifidobacterium species [113, 117]. B. thetaiotaomicron can utilize mucin glycoproteins to liberate free sialic acid, but it cannot further utilize sialic acid. In contrast, C. difficile is able to use sialic acid as a nutrient for growth, mainly relying on the sialic acid metabolic pathway [118]. Another classical example is the production of putrescine. Putrescine is synthesized from agmatine, which is produced through the decarboxylation of arginine by the gut microbiota. In most cases, two metabolic pathways can be used to convert agmatine to putrescin: the agmatine ureohydrolase (SpeB) pathway and the N-carbamoylputrescine pathway. However, Kitada et al., found a novel putrescine synthesis pathway that requires the cooperation of two different bacterial species, neither of which express a complete agmatine biosynthetic pathway. Briefly, one species (such as E. coli) converts arginine to agmatine, and the other species (such as Enterococcus faecalis) yields putrescine from agmatine via agmatine deaminase [119]. More generally, cross-feeding of breakdown products in the gut environment is critical for various gut bacterial inhabitants.
Syntrophic cross-feeding for interspecies metabolic exchanges is very common in natural communities, and such exchanges offer significant advantages under poor nutrient conditions [112]. This cross-feeding can also balance cooperative interactions in the gut microbiota and play an important role in regulating intestinal nutrient absorption [120].
Metabolites and the host immune system
The intestinal microbiota helps to regulate many host physiological processes, such as nutritional homeostasis, energy expenditure, and immunity [121]. In recent years, several studies have revealed that the microbiota plays a critical role in host immune development and immune regulation. This intimate crosstalk may be driven by the secretion and signaling of small molecule metabolites and has profound impacts on host immunity and physiology [122]. As mentioned above, many microbiota-associated metabolites are bioactive, such as SCFAs, indole, and secondary bile acids, and these metabolites can react with corresponding sensing platforms in the host through signaling pathways [123] (Fig. 3).
Effects of metabolites on immune cells. Metabolites derived from the microbiota or host participate in complicated host-microbiota interactions. SCFAs short chain fatty acids, AMPs antimicrobial peptides, AhR aryl hydrocarbon receptor, FXR farnesoid X receptor, PXR pregnane X receptor, HADC histone deacetylase, TJ tight junction, ILC3 group 3 innate lymphoid cell
Bioactive metabolites affect the maturation, activation, polarization, and effector functions of innate and adaptive immune cells, thereby modulating anti-inflammatory or pro-inflammatory responses (Table 2). In the next sections, we will provide an overview of the effects of microbiota-associated metabolites on intestinal immune cell subsets and their functions.
Interaction between metabolites and innate immunity
Intestinal epithelial cells (IECs)
The intestinal tract plays a key role in protecting the host from the environment and mediating nutrient absorption. The intestinal surface is composed of IECs, which form an important physical barrier that separates the lumen from the LP of intestinal and commensal bacteria [131]. Although IECs are not classical innate immune cells, they are a critical part of the mucosal immune system. Indeed, IECs are equipped with an extensive repertoire of innate immune receptors, which contribute to gut homeostasis through bacterial recognition [160].
SCFAs are the products of nondigestible carbohydrate fermentation by the intestinal microbiota. The host recognizes SCFAs by several G-protein-coupled-receptors (GPR41, GPR43 and GPR109A) [148, 161]. SCFAs serve as energy substrates for IECs, especially butyrate, which can modulate energy metabolism in IECs [124]. However, butyrate functions as not only as an energy source for colonocytes, but as also an inhibitor of intestinal stem cells. Butyrate suppresses proliferation by inhibiting histone deacetylase (HDAC) and increasing the promoter activity of cell-cycle negative regulators [125]. Therefore, the utilization of butyrate by enterocytes limits intestinal stem cell access to metabolites and protects these cells from anti-proliferative effects. SCFAs promote the transcription of mucin genes in epithelial goblet cells, and inoculation of several SCFA-producing bacteria in germ-free mice induces the differentiation of goblet cells and secretion of mucus [126, 162].
The inflammasome is a critical innate immunity sensing complex of IECs that regulates interactions between the host and microbiota by producing cytokine IL-18 and downstream antimicrobial peptides and secreting mucus [127, 163]. Butyrate influences the NLRP3 inflammasome by activating GPR43 or GPCR109A in IECs, facilitating the expression of the downstream inflammatory cytokine IL-18 and thus promoting epithelial repair and maintaining barrier function [121, 127]. Activation of the NLRP6 inflammasome is regulated by taurine, histamine, and spermine, whereas spermine and histamine can inhibit NLRP6 inflammasome signaling [128, 164].
Additionally, secondary bile acids (DCA and LCA) regulate epithelial cell integrity and microorganism composition by binding to the nuclear receptor farnesoid X receptor (FXR) expressed in IECs [129]. Mice with FXR deficiency exhibit epithelial barrier disruption and imbalanced gut homeostasis [130]. Indole, an amino acid microbial-mediated metabolite, promotes epithelial barrier function through the pregnane X receptor (PXR), which contributes to the reinforcement of tight junctions. By increasing the production of occludin, zonula occludens 1 (ZO1) and E-cadherin, polyamines are also responsible for enhancing the integrity of the IEC barrier [132, 165].
Innate lymphoid cells (ILCs)
Compared with adaptive lymphocytes, ILCs are relatively rare innate lymphocytes, but they are abundant on the barrier surface of mucosal-associated tissues [166, 167]. Recently, several studies reported that specific microbial metabolites can regulate ILCs, and most research has focused on ILC3 [168, 169], which produces the cytokine IL-22, a member of the IL-10 cytokine family. Lack of IL-22 is associated with different host pathologies (infections and metabolic disorders) [133, 170]. Furthermore, IL-22 promotes the expression of antimicrobial peptides (RegIIIγ and RegIIIβ) to limit colonization by commensal bacteria (such as SFBs), induces the fucosylation of surface proteins to promote the colonization of beneficial bacteria, and enhances the proliferation of goblet cells for mucin secretion [134,135,136].
AhR is a ligand-dependent transcription factor that plays a critical role in mucosal immunity. AhR ligands bind to the ligand-binding domain of the receptor, promoting conformational remodeling that exposes the nuclear localization sequence, and facilitates the transcription of target genes. The nuclear receptor RORγt is critical for the development of ILCs and RORγt+ ILCs are abundantly present in the intestinal LP [171,172,173]. In fact, without AhR, RORγt+ ILCs undergo higher rates of apoptosis and produce less IL-22. RORγt interacts with AhR to stimulate the binding of the latter at the IL-22 locus, promoting transcription [174]. Tryptophan catabolites can specifically regulate ILC3. As indicated above, tryptophan can be converted to active substances by both microorganisms and hosts, and most of these metabolites are AhR agonists (Fig. 1). Tryptophan acts as a metabolic substrate for the production of AhR ligands, such as indole-3-aldehyde and indole-3-lactic acid, by members of Lactobacillus, and these ligands help to resist colonization by C. albicans and SFBs[65]. For instance, the Lactobacillus population in the Card9−/− mouse intestine was shown to be low, which led to impairment of tryptophan metabolism and thus decreased AhR ligand production. Such a defect further impairs IL-22 production and increases the susceptibility of mice to dextran sodium sulfate (DSS)-induced colitis [91]. In addition, the ability to produce AhR agonists, especially those derived from the microbiota, in the feces of irritable bowel disease (IBD) patients is impaired. In general, the balance of ILC3 populations and IL-22 production is deeply dependent on the ability to produce AhR agonists; accordingly, these endogenous microbe-derived tryptophan metabolites are crucial for resistance to colonization and maintenance of gut immune homeostasis.
Dendritic cells (DCs)
DCs are professional antigen-presenting cells that play a key role in bridging the innate and adaptive immune system. The immunomodulatory effects of DCs depend on producing cytokines and inducing the polarization and differentiation of naive CD4+ T lymphocytes [175]. The activation, survival, and maturation of DCs are processes that are influenced by mainly local factors within their microenvironment, such as microbial components, cytokines, and metabolites [176].
SCFAs act as HDAC inhibitors, and when exposed to DCs, SCFA-driven HDAC suppression is critical for inhibiting nuclear factor-κB (NF-κB) and tumor necrosis factor-α (TNF-α) [177, 178]. Propionate and butyrate inhibit bone-marrow precursor differentiation into DCs via their transporter Slc5a8 [137, 138], and exposure of DCs to butyrate is accompanied by decreased production of the inflammatory cytokines IL-12 and IFN-γ, and increased production of IL-10 and IL-23 [179]. Some evidence also indicates that butyrate may modulate the ability of DCs to present antigens and induce T cell differentiation. For instance, treatment of DCs with butyrate reduced the expression of co-stimulatory proteins, including CD40, CD80, CD86 and major histocompatibility complex class II molecules [139].
Histamine is a biogenic amine converted from the amino acid histidine via decarboxylase (hdc) (Table 1) [147]. Histamine regulates the responses of DCs to microbial ligands through H2R and then enhances the DC antigen-presenting capacity [140]. Administration of a histamine-producing Lactobacillus rhamnosus strain to H2R-knockout mice reduces the microbe’s immunoregulatory activities[84]. Additionally, L. reuteri-derived histamine suppresses the production of TNF-α and inhibits MEK/extracellular signal-regulated kinase mitogen-activated protein kinase (MAPK) signaling.
Secondary bile acids act as agonists of G-protein-coupled bile acid receptor 1 (GPBAR1) and nuclear receptor subfamily 1, group H, member 4 (NR1H4; also known as FXR), regulating the function of DCs [180, 181]. These receptors are linked to anti-inflammatory responses, including inhibition of NF-κB activity and NF-κB-dependent transcription [141, 182]. GPBAR1 signaling leads to the cAMP-PKA-regulated suppression of STAT1 and NF-κB, and NR1H4 signaling involves NR1H4-NCoR1-mediated repression of NF-κB-responsive elements (NRE) [183]. Activation of GPBAR1 leads to intracellular cAMP accumulation, further promoting the differentiation of CD14+ monocytes into CD209+ DCs [184].
Neutrophils
Neutrophils are the first effector cells that accumulate at an inflammation site, where they kill and digest bacteria [185]. In the case of mucosal infection or inflammation, neutrophils will pass through the LP and enter infected or inflamed sites. The migration of neutrophils depends on multiple factors, such as chemokines and integrins [186, 187]. After migrating to the designated location, activated neutrophils produce nitric oxide (NO), chemokines (e.g., IL-8) and pro-inflammatory factors (e.g., TNF-α and IL-1β), leading to maintenance of the inflammatory response as well as mucosal injury [188].
Several reports have shown that SCFAs are effective activators of neutrophils [148, 188, 189], as in vitro experiments have demonstrated that SCFAs induce neutrophil chemotaxis by binding to GPR43. In turn, GPR43-dependent signaling leads to the phosphorylation and activation of p38 MAPK [188], and p38 MAPK phosphorylation has been described as the major determinant of chemotaxis [142]. Compared with normal bone marrow-derived neutrophils (BMDNs), Gpr43−/− BMDNs do not exhibit calcium influx and chemotaxis when treated with acetate [190]. Furthermore, Gpr43−/− colitis mice treated with SCFAs (acetate or butyrate) showed reduced colonic neutrophil contents compared with those of wild-type colitis mice [191]. The inhibition of HDAC activity by SCFAs also suppress of NF-κB activation and NO production in neutrophils. Similar results were observed after exposure to HDAC inhibitors [143]. Nonetheless, SCFA specificity exists in reactive oxygen species (ROS) production, with acetate promoting production, butyrate inhibiting production, and propionate having no effect [144, 145]. The ROS produced by neutrophils help to protect against microbiota, modulate intracellular signaling activity, and regulate the inflammatory process [192, 193].
Interactions between metabolites and adaptive immunity
The effects of commensal microbiota on the immune system include not only innate immunity but also adaptive immune responses. For example, germ-free mice have an immature adaptive immune system [28]. Although the molecular mechanism responsible for the effect of the microbiota on immune system development is still largely unknown, several metabolites have been shown to regulate adaptive immune cell function, especially those of CD4+ T and B lymphocytes.
CD4+ T lymphocytes
As we previously discussed above, individual commensal species help to modulate polarization of the CD4+ T-cell compartment to defined CD4+ T cell subsets, including Th cells (Th1, Th2, Th17 cells) and Tregs. In generally, immune homeostasis and tolerance in the gut constitute the balance between pro-inflammatory effector Th cells and anti-inflammatory Tregs.
T helper (Th) cells
IFN-γ secretion by antigen presenting cells acts on original Th-cell signal transduction and transcription activating factor 1 (STAT-1). STAT-1 activates the specific transcription factor T-bet and prompts the differentiation of original Th cells into Th1 cells. Thl and Th2 cells are dynamically balanced under normal conditions, and are regulated and inhibited by cytokines secreted by each other. IFN-γ secreted by Th1 cells inhibits the polarization of Th2 cells, while IL-4 produced by Th2 cells inhibits Th1 proliferation. The imbalance of Th1/Th2 cells is associated with a variety of autoimmune diseases and inflammatory diseases, such as IBD [194]. The activation of different receptors in cell surface or intracellular could cause different activities. For example, the activation of H1R on lymphocytes promotes TH1 polarization, while the activation of H2R suppresses TH1 and TH2 polarization [147]. Histamine has important regulatory effects on T lymphocytes that are dependent on receptors.
Th17 cells are a CD4+ T-cell polarization in addition to Th1 and Th2, which are also pro-inflammatory effector Th cells. Th17 cells mainly function through secreting the cytokines, IL-17, IL-23 and IL-22, and pro-inflammatory mediators IL-17 and IL-23 can activate NF-κB and related inflammatory signaling pathway as well as block the expression of the anti-inflammatory cytokines IL-10 [195]. AhR expression is reduced in pathogenic Th17 cells, which leads to tissue inflammation. The AhR ligand indole-3-lactic acid was shown to inhibit the polarization of mouse Th17 cells in vitro [146]. In addition to the direct regulation of CD4+T cells function by microbial metabolites, the cytokines produced by IECs, DCs or macrophages induced by microbiota signals also play a significant role in CD4+ T-cell functionality [196, 197].
Regulatory T (Treg) cells
In the intestinal LP, many unique lymphocytes operate cooperatively to defend against infectious pathogens and maintain the epithelial mucosal barrier. However, an unrestrained inflammatory response to the ingestion of food and resident commensal microbiota by effector T cells or myeloid cells is harmful to intestinal health and immune homeostasis [198]. To maintain immune homeostasis, the immune system must tolerate antigens derived from food and gut microbiota, which partially depend on inducible Tregs. Tregs are characterized by the expression of CD4, CD25 and Foxp3 as well as the production of the anti-inflammatory cytokines TGF-β and IL-10. The development of intestinal Treg cells is affected by several metabolites derived from the microbiota and host.
As mentioned above, SCFAs have many effects on the innate immune system, and profound impact Treg biology. Several studies have demonstrated that SCFAs affect the number of Foxp3+/IL-10+ colonic Tregs and enhance the regulation of Tregs in the large intestine [149]. Further research has revealed that SCFAs stimulate the proliferation of Tregs by activating GPR43 or GPR41 [103] and the differentiation of naive CD4+ T cells into Tregs by inhibiting HDAC (e.g., HDAC6 and HDAC9) activation [149]. HDAC suppression in T cells enhances the acetylation of p70 S6 kinase and phosphorylation of rS6, and further regulates the mTOR pathway associated with the generation of IL-10 + T cells [150]. Interestingly, different SCFAs modulate Tregs through different mechanisms; acetate and propionate stimulate the expansion of preexisting colonic Treg cells (cTregs), whereas butyrate increases the de novo differentiation of naïve T cells toward Tregs [199]. In addition to directly regulating the responses of T cells, SCFAs modulate DC-T cell interactions, e.g., inhibiting the expression of the NF-κB component RelB through HDAC inhibition and inducing anti-inflammatory genes through GPR109A activation in DCs, thereby resulting in Tregs differentiation [164].
Retinoic acid (RA), a metabolite of dietary vitamin A catalyzed via aldehyde dehydrogenase (ALDH), plays a crucial role in mediating Treg expansion [200, 201]. In combination with TGFβ, RA induces the differentiation of peripherally derived Treg cells (pTregs) [202, 203]. In addition, by binding to RA receptor (RAR) and retinoid X receptor (RXR) heterodimers, RA activates TGFβ–SMAD signaling to promote Foxp3 transcription [151, 152]. RA also enhances the generation of RORγt+Foxp3+ pTregs in vitro, and inhibiting RA signaling prevents the development of these cells in vivo [204, 205]. RA increases the expression of gut-homing markers (e.g., CCR9 and α4β7 integrin) on pTregs, which helps naive T cells migrate to various tissue sites [202, 206, 207]. Other vitamins that regulate interactions between the gut microbiota and host mostly belong to the B and K groups, which the host cannot synthesize and must depend on commensal microorganisms for production [208, 209]. For instance, the water-soluble vitamin B9 (folic acid), which plays a critical role in maintaining Foxp3+ Treg cell homeostasis, can be synthesized by several bacteria (e.g., Bifidobacterium and Lactobacillus) [210,211,212,213]. FA promotes the survival of Foxp3+ Tregs by upregulating the expression of the antiapoptotic factor BCL-2. Mice fed an FA-deficient diet exhibit a reduced number of Foxp3+ Tregs in the intestinal LP and a higher susceptibility to colitis [153, 154].
As discussed above, most tryptophan metabolites are AhR ligands. However, some host-derived tryptophan metabolites (such as KA and niacin) can also act as GPR35 and GPR109A agonists to induce colonic Treg differentiation [155]. The L. reuteri-derived AhR agonist indole-3-lactic acid reprograms intraepithelial CD4+ T cells into CD4+ CD8αα+ T cells [156]. Polyamines have also been reported to be important for accelerating the maturation of LP CD4+ T cells to regulate adaptive immunity in rats [199, 214, 215]. In addition to its effects on the cytokine secretion of DCs, histamine also influences the function of Tregs depending on the receptors they express [216]; for example, the activation of H1R expressed on Tregs leads to inhibition of the suppressive functions of these cells, as related to the decreased expression of CD25 and Foxp3[217].
B cells
Immunoglobulin secretion plays a critical role in immune regulation and protection against microorganisms in the intestine. Secretory immunoglobulin A (SIgA) populations comprise the largest class of immunoglobulins in the intestinal mucosal [33]. SIgA is secreted by plasma cells (differentiated B cells) in the LP and then passes through the intestinal epithelium into the lumen, where it targets microbial antigens and prevents bacterial translocation and infection [16].
Dietary fiber intake is positively correlated with intestinal IgA levels [218, 219]. One study revealed that SCFAs are able to regulate B-cell gene expression via HDAC inhibition to promote antibody secretion, similar to the effect of SCFAs on other cells [157]. Further research has shown that SCFAs modulate metabolic sensors to enhance oxidative phosphorylation, glycolysis and fatty acid synthesis in B cells [158]. These functions increase mitochondrial energy production and the levels of building blocks to promote B-cell activation, differentiation, and antibody production [220]. RA also modulates the activity of B cells, directly affecting their ability to facilitate IgA class-switch recombination and IgA production [159]. Nonetheless, as the role of microbial metabolites in the regulation of antibody production in B cells remains largely unclear, more research on the regulation of commensally derived metabolites of host antibody responses is needed.
Microbiota-gut-brain axis: maintenance of immune homeostasis
The gut and brain are intimately connected by the gut-brain axis, which is a complex bidirectional adjustment system that includes the central and enteric nervous systems [221, 222]. Signals from the brain, and vice versa, influence the sensory and secretory modalities of the gastrointestinal tract, regulate the inflammatory process and influence the gut microbiota structure [223]. In turn, messages secreted from the gastrointestinal tract can influence brain function. A dysbiotic microbiota (associated with a high-sugar diet) reportedly alters vagal gut-brain communication [224]. The immune system plays a vital role in gut-brain axis communication, as immune mediators are important messengers in this complex dialog, which may explain the functional impairments in both the brain and gut in some diseases (e.g., IBD and psychological morbidity) [225].
As previously described, the bioactive small molecule metabolites originating from the gut microbiota can maintain intestinal immune homeostasis dependent on lymphocytes. In recent years, increasing evidence supports that these microbial products can enter the circulatory system and prominently affect the inflammatory responses of resident cells in the central nervous system (CNS). While SCFAs have immunomodulatory effects on the gut, they can also permeate the mucosa into the LP and enter systemic circulation [226]. Acetate and propionate, but not butyrate, can be detected in the peripheral circulation, indicating that distinct SCFAs have different functions in the immune system [227, 228]. SCFAs can also directly signal to the CNS depending on stimulation of the vagus nerve or exert indirect effects through immune-neuroendocrine processes [229]. Systemic SCFAs are capable of increasing the integrity of the blood–brain barrier by upregulating of the tight junction protein occludin [230]. Prebiotics such as inulin increased the production of acetate by crossing the blood–brain barrier in rats and affecting physiological function [231]. Tryptophan metabolites produced by the microbiota suppress NF-κB signaling activation, TGFα and VEGF-B production in microglia via activating AhR [232]. Lower VEGF-B expression limits the transcriptional programming of inflammatory astrocytes and thus limits their ability to produce damaging metabolites, such as NO, which recruit peripheral immune cells, and activate T and B cells in the CNS, thus amplifying local inflammation [196]. Indole, indoxyl-3-sulfate, indole-3-propionic acid and indole-3-aldehyde cross the blood–brain barrier and suppress inflammation in combination with IFN-I in astrocytes [13].
Concluding remarks
Scientific research has revealed the complexity and breadth of interactions between commensal microorganisms and hosts. Metabolites derived by the microbiota serve as chemical messengers that mediate crosstalk between the microbes and host and play beneficial and deleterious roles in host health. In fact, the intestinal microbiota appears to affect many human disorders (e.g., IBD, cancer and allergies) [233].
Identifying the molecular mechanisms by which the gut microbiota or microbial metabolites modulate host health is important for exploring new approaches for disease research. However, research on the regulation of host-microbe interactions by metabolites has focused on only certain aspects, such as intestinal immunity and microbial composition, and the in-depth molecular mechanism remains unclear. Small molecule metabolites, such as indole, also act as signaling molecules for interbacterial communication and quorum sensing, thereby driving changes in the function and composition of the microbiota to modulate intestinal homeostasis. Overall, interactions between the host and the microbiota are complex and variable. The following questions need to be answered: (1) Can metabolites originate from the host, the microbiota or their common regulation, and how can these sources be distinguished? (2) Can some factors (e.g., diet, age, environment, and mental health) affect gut microbiota composition, and in turn affect the production of microbial metabolites? (3) What are the structures and metabolic synthesis mechanisms of these unknown biologically active microbial metabolites? (4) What interactions exist between multiple metabolites from different microbiota?
Fortunately, new approaches (e.g., multiomics approaches) have begun to unravel these complex host-microbiota interactions and further our knowledge of the mechanism involved. To complement traditional metabolomics, metagenomics helps to elucidate the mechanisms underlying metabolites biosynthesis through a bioinformatics perspective and discover more new biologically active microbial metabolites [233].
Using small molecules from microbiota as drugs to reduce inflammation and disease severity is promising but basic research and clinical data with complex traits are still required. Additionally, because of microbiota and genetic differences between individuals, personalized precision medicine should be considered [234].
Thus, a deeper understanding of the effects of metabolites on host homeostasis and disease may provide an opportunity for the administration of small molecule metabolites as prophylactic or therapeutic treatments in microbiota-related diseases.
References
Hooper LV (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–884
Stockinger B, Di Meglio P, Gialitakis M, Duarte JH (2014) The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32:403–432
Round JL, Palm NW (2018) Causal effects of the microbiota on immune-mediated diseases. Sci Immunol 3:o1603
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16:341–352
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141
Manichanh C, Borruel N, Casellas F, Guarner F (2012) The gut microbiota in IBD. Nat Rev Gastro Hepat 9:599–608
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267
Tremaroli V, Bäckhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249
Zheng X, Xie G, Zhao A, Zhao L, Yao C, Chiu NHL, Zhou Z, Bao Y, Jia W, Nicholson JK, Jia W (2011) The footprints of gut microbial-mammalian co-metabolism. J Proteome Res 10:5512–5522
Hill DA, Artis D (2010) Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol 28:623–667
Hooper LV, Dan RL, Macpherson AJ (2012) Interactions between the microbiota and the immune system. Science 336:1268–1273
Chinen T, Rudensky AY (2012) The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev 245:45–55
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44:450–462
Belkaid Y, Bouladoux N, Hand TW (2013) Effector and memory T cell responses to commensal bacteria. Trends Immunol 34:299–306
Macpherson AJ, Uhr T (2004) Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662–1665
Postler TS, Ghosh S (2017) Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26:110–130
Bauer H, Horowitz RE, Levenson SM, Popper H (1963) The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol 42:471–483
Falk PG, Hooper LV, Midtvedt T, Gordon JI (1998) Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol R 62:1157–1170
Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu HJ, Kasper DL, Benoist C, Mathis D (2016) Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci 113:E8141–E8150
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K (2011) Induction of colonic regulatory T cells by indigenous clostridium species. Science 331:337–341
Vázquez-Castellanos JF, Serrano-Villar S, Jiménez-Hernández N, Soto Del Rio MD, Gayo S, Rojo D, Ferrer M, Barbas C, Moreno S, Estrada V, Rattei T, Latorre A, Moya A, Gosalbes MJ (2018) Interplay between gut microbiota metabolism and inflammation in HIV infection. ISME J 12:1964–1976
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–236
Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, Benoist C, Kasper DL (2017) Mining the human gut microbiota for immunomodulatory organisms. Cell 168:928–943
Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA (2014) Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158:1000–1010
Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, Chiavelli A, D’Erchia AM, Sethi MK, Pabst O, Marzano M, Moretti S, Romani L, Penna G, Pesole G, Rescigno M (2015) BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity 43:527–540
Kubinak JL, Round JL (2016) Do antibodies select a healthy microbiota? Nat Rev Immunol 16:767–774
Smith K, McCoy KD, Macpherson AJ (2007) Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19:59–69
Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118
Talham GL, Jiang HQ, Bos NA, Cebra JJ (1999) Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun 67:1992–2000
Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, Ermund A, Boysen P, Bemark M, Sommer F, Backhed F, Hansson GC, Johansson ME (2015) The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16:164–177
Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci 105:15064–15069
Rossi O(2013)Interactions of commensal bacteria with the host immune system(Vol
Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG (2011) Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to clostridium difficile-induced colitis. Infect Immun 80:62–73
Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MRM, Kamboj M, Pamer EG (2010) Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341
Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G (2008) Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456:507–510
Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG (2008) Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455:804–807
Kamada N, Núñez G (2014) Regulation of the immune system by the resident intestinal bacteria. Gastroenterology 146:1477–1488
Cording S, Fleissner D, Heimesaat MM, Bereswill S, Loddenkemper C, Uematsu S, Akira S, Hamann A, Huehn J (2013) Commensal microbiota drive proliferation of conventional and Foxp3 + Regulatory CD4 + T cells in mesenteric lymph nodes and Peyer’s patches. Eur J Microbiol Immunol 3:1–10
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498
Geng S, Cheng S, Li Y, Wen Z, Ma X, Jiang X, Wang Y, Han X (2018) Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. J Crohn’s Colitis 12:1359–1374
Fischer M, Sipe BW, Rogers NA, Cook GK, Robb BW, Vuppalanchi R, Rex DK (2015) Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: description of a protocol with high success rate. Aliment Pharm Ther 42:470–476
Shen Z, Zhu C, Quan Y, Yang Z, Wu S, Luo W, Tan B, Wang X (2018) Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroentero 24:5–14
Ekmekciu I, von Klitzing E, Fiebiger U, Escher U, Neumann C, Bacher P, Scheffold A, Kühl AA, Bereswill S, Heimesaat MM (2017) Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front Immunol 8:397
Ekmekciu I, von Klitzing E, Neumann C, Bacher P, Scheffold A, Bereswill S, Heimesaat MM (2017) Fecal microbiota transplantation, commensal Escherichia coli and Lactobacillus johnsonii strains differentially restore intestinal and systemic adaptive immune cell populations following broad-spectrum antibiotic treatment. Front Microbiol 8:2430
Cummings JH, Macfarlane GT (2016) Collaborative JPEN-clinical nutrition scientific publications role of intestinal bacteria in nutrient metabolism. Jpen-Parenter Enter 21:357–365
Matsumoto M, Kibe R, Ooga T, Aiba Y, Kurihara S, Sawaki E, Koga Y, Benno Y (2012) Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep-Uk 2:233
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci 106:3698–3703
Miller TL, Wolin MJ(1996)Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora 62:1589
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nature Rev Microbiol 12:661–672
Louis P, Duncan SH, Mccrae SI, Millar J, Jackson MS, Flint HJ (2004) Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon 186:2099
Reichardt N, Duncan SH, Young P, Belenguer A, Mcwilliam LC, Scott KP, Flint HJ, Louis P (2014) Phylogenetic distribution of three pathways for propionate production within the human gut microbiota 8:1323–1335
Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ (2002) Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microb 68:5186–5190
Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ (2000) Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microb 66:1654–1661
Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown DS, Stares MD, Scott P, Bergerat A (2011) Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5:220–230
Louis P, Young P, Holtrop G, Flint HJ (2010) Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol 12:304–314
Christiansen N, Ahring BK (1996) Introduction of a de novo bioremediation activity into anaerobic granular sludge using the dechlorinating bacterium DCB-2. Anton Leeuw Int J G 69:61–66
Elsden SR, Hilton MG, Waller JM (1976) The end products of the metabolism of aromatic amino acids by clostridia. Arch Microbiol 107:283–288
DeMoss RD, Moser K (1969) Tryptophanase in diverse bacterial species. J Bacteriol 98:167–171
Pickett MJ (1989) Methods for identification of flavobacteria. J Clin Microbiol 27:2309–2315
Smith EA, Macfarlane GT (2010) Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Microbiol 81:288–302
Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL (2017) A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551:648–652
Wlodarska M, Luo C, Kolde R, Hennezel ED, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA (2017) Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 22:25–37
Honoré AH, Aunsbjerg SD, Ebrahimi P, Thorsen M, Benfeldt C, Knøchel S, Skov T (2016) Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal Bioanal Chem 408:83–96
Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, Angelo CD, Massibenedetti C, Fallarino F (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385
Aragozzini F, Ferrari A, Pacini N, Gualandris R (1979) Indole-3-lactic acid as a tryptophan metabolite produced by Bifidobacterium spp. Appl Environ Microb 38:544–546
Whitehead TR, Price NP, Drake HL, Cotta MA (2008) Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl Environ Microb 74:1950–1953
Honeyfield DC, Carlson JR (1990) Effect of indoleacetic acid and related indoles on Lactobacillus sp. strain 11201 growth, indoleacetic acid catabolism, and 3-methylindole formation. Appl Environ Microb 56:1373–1377
Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA (2014) Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16:495–503
Ridlon JM, Hylemon PB (2012) Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7α-dehydroxylating intestinal bacterium. J Lipid Res 53:66–76
Lee J, Arai H, Nakamura Y, Fukiya S, Wada M, Yokota A (2013) Contribution of the 7β-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J Lipid Res 54:3062
Jones BV, Begley M, Hill C, Cormac GMG, Marchesi JR (2008) Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA 105:13580–13585
Lepercq P, Gérard P, Béguet F, Raibaud P, Grill J, Relano P, Cayuela C, Juste C (2004) Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by Clostridium baratii isolated from human feces. FEMS Microbiol Lett 235:65–72
Tazuke Y, Matsuda K, Adachi K, Tsukada Y (1998) Purification and properties of a novel sulfatase from Pseudomonas testosteroni that hydrolyzed 3β-hydroxy-5-cholenoic acid 3-sulfate. Biosci Biotechnol Biochem 62:1739–1744
Charles N, Deborah P, Eric V, Christophe M, Thierry G, Eric Q, Pieter D, Valérie S, Philippe G, Jacques D (2010) Control of acute, chronic, and constitutive hyperammonemia by wild-type and genetically engineered Lactobacillus plantarum in rodents. Hepatology 48:1184–1192
Jia W, Xie G, Jia W (2017) Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastro Hepat 15:111
Kitahara M, Takamine F, Imamura T, Benno Y (2001) Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity. Int J Syst Evol Micr 51:39
Hirano S, Masuda N (1981) Transformation of bile acids by Eubacterium lentum. Appl Environ Microb 42:912–915
Hirano S, Masuda N, Oda H, Mukai H (1981) Transformation of bile acids by Clostridium perfringens. Appl Environ Microb 42:394–399
Devlin AS, Fischbach MA (2015) A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol 11:685–690
Pegg AE (2013) Toxicity of polyamines and their metabolic products. Chem Res Toxicol 26:1782–1800
Di Martino ML, Campilongo R, Casalino M, Micheli G, Colonna B, Prosseda G (2013) Polyamines: emerging players in bacteria–host interactions. Int J Med Microbiol 303:484–491
Kadioglu A, Weiser JN, Paton JC, Andrew PW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6:288
Masaki T, Yoshimatsu H (2006) The hypothalamic H1 receptor: a novel therapeutic target for disrupting diurnal feeding rhythm and obesity. Trends Pharmacol Sci 27:279–284
Flint HJ, Duncan SH, Scott KP, Louis P (2015) Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 74:13–22
Levy M, Thaiss CA, Elinav E (2016) Metabolites: messengers between the microbiota and the immune system. Gene Dev 30:1589–1597
Louis P, Scott KP, Duncan SH, Flint HJ (2010) Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102:1197–1208
Bobik TA, Havemann GD, Busch RJ, Williams DS, Aldrich HC (1999) The propanediol utilization (pdu) operon of Salmonella enterica serovar typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J Bacteriol 181:5967–5975
Scott KP, Martin JC, Campbell G, Mayer CD, Flint HJ (2006) Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J Bacteriol 188:4340–4349
Ríoscovián D, Ruasmadiedo P, Margolles A, Gueimonde M, Cg RG, Salazar N (2016) Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7:185
Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da CG, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM (2016) CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22:598–605
Yokoyama MT, Carlson JR (1979) Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am J Clin Nutr 32:173
Smith EA, Macfarlane GT (1997) Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microb Ecol 33:180–188
Li G, Young KD (2013) Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 159:402–410
Keszthelyi D, Troost FJ, Masclee A (2009) Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroent Motil 21:1239–1249
Cook KL, Rothrock MJ, Loughrin JH, Doerner KC (2010) Characterization of skatole-producing microbial populations in enriched swine lagoon slurry. FEMS Microbiol Ecol 60:329–340
Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C (2014) Aryl hydrocarbon receptor control of a disease tolerance defense pathway. Nature 511:184–190
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197
Adams S, Braidy N, Bessesde A, Brew BJ, Grant R, Teo C, Guillemin GJ, Adams S, Braidy N, Bessesde A (2012) The kynurenine pathway in brain tumor pathogenesis. Cancer Res 72:5649–5657
Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104:979–984
Wahlström A, Sayin SI, Marschall HU, Bäckhed F (2016) Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24:41–50
Ridlon JM, Kang DJ, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259
Turroni S, Brigidi P, Cavalli A, Candela M (2017) Microbiota-host transgenomic metabolism, bioactive molecules from the inside. J Med Chem 61:47–61
Doerner KC, Takamine F, Lavoie CP, Mallonee DH, Hylemon PB (1997) Assessment of fecal bacteria with bile acid 7 alpha-dehydroxylating activity for the presence of bai-like genes. Appl Environ Microb 63:1185–1188
Midtvedt T (1974) Microbial bile acid transformation. Am J Clin Nutr 27:1341–1347
Kisiela M, Skarka A, Ebert B, Maser E (2012) Hydroxysteroid dehydrogenases (HSDs) in bacteria: a bioinformatic perspective. J Steroid Biochem Mol Biol 129:31–46
Fukiya S, Arata M, Kawashima H, Yoshida D, Kaneko M, Minamida K, Watanabe J, Ogura Y, Uchida K, Itoh K (2009) Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. FEMS Microbiol Lett 293:263–270
Beuers U, Fischer S, Spengler U, Paumgartner G (1991) Formation of iso-ursodeoxycholic acid during administration of ursodeoxycholic acid in man. J Hepatol 13:97–103
Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 307:1915–1920
Sender R, Fuchs S, Milo R (2016) Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164:337–340
Pande S, Shitut S, Freund L, Westermann M, Bertels F, Colesie C, Bischofs IB, Kost C (2015) Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat Commun 6:6238
Zelezniak A, Andrejev S, Ponomarova O, Mende DR, Bork P, Patil KR (2015) Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc Natl Acad Sci USA 112:6449–6454
Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ (2006) Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microb 72:3593–3599
Flint HJ, Duncan SH, Scott KP, Louis P (2007) Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol 9:1101–1111
Marquet P, Duncan SH, Chassard C, Bernalierdonadille A, Flint HJ (2010) Lactate has the potential to promote hydrogen sulphide formation in the human colon. FEMS Microbiol Lett 299:128–134
Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, Michel C (2005) Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol 99:201–212
Falony G, Vlachou A, Verbrugghe K, De VL (2006) Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microb 72:7835
Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM (2013) Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502:96
Kitada Y, Muramatsu K, Toju H, Kibe R, Benno Y, Kurihara S, Matsumoto M (2018) Bioactive polyamine production by a novel hybrid system comprising multiple indigenous gut bacterial strategies. Sci Adv 4:t62
Foster KR, Bell T (2012) Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol 22:1845–1850
Levy M, Blacher E, Elinav E (2017) Microbiome, metabolites and host immunity. Curr Opin Microbiol 35:8–15
Donia MS, Fischbach MA (2015) Small molecules from the human microbiota. Science 349:1254766
Thorburn Alison, Nbsp Macia, Laurence Mackay, Charles Nbsp (2014) Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40:833–842
Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13:517–526
Kaiko G, Ryu S, Koues O, Collins P, Solnica-Krezel L, Pearce E, Pearce E, Oltz E, Stappenbeck T (2016) The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165:1708–1720
Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TM, Palaniyar N, Grasemann H (2015) Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 46:1033–1045
Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilbermanschapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y (2015) Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163:1428–1443
Chang PV, Hao L, Offermanns S, Medzhitov R (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111:2247–2252
Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553
Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ (2006) Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 103:3920–3925
Thaiss CA, Zmora N, Levy M, Elinav E (2016) The microbiome and innate immunity. Nature 535:65
Chen J, Rao JN, Zou T, Liu L, Marasa BS, Xiao L, Zeng X, Turner DJ, Wang JY (2007) Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 293:G568–G576
Wang X, Ota N, Manzanillo P, Kates L, Zavalasolorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J (2014) Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514:237–241
Sonnenberg GF, Artis D (2015) Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med 21:698
Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV (2013) Nonredundant function of soluble LTÎ ± 3 produced by innate lymphoid cells in intestinal homeostasis. Science 342:1243–1246
Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345:1254009
Wang B, Morinobu A, Horiuchi M, Liu J, Kumagai S (2008) Butyrate inhibits functional differentiation of human monocyte-derived dendritic cells. Cell Immunol 253:54–58
Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J (2010) Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol 130:245–255
Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, Veniaminova NA, Merchant JL, Chen CC, Huffnagle GB (2012) Butyrate increases IL-23 production by stimulated dendritic cells. Am J Physiol-Gastr L 303:G1384
Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM (2001) Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest 108:1865
Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S (2009) The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 183:6251–6261
Capodici C, Hanft S, Feoktistov M, Pillinger MH (1998) Phosphatidylinositol 3-kinase mediates chemoattractant-stimulated, CD11b/CD18-dependent cell-cell adhesion of human neutrophils: evidence for an ERK-independent pathway. J Immunol 160:1901–1909
Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R (2011) Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22:849–855
Segal AW (2005) How neutrophils kill microbes. Annu Rev Immunol 23:197–223
Nakao S, Fujii A, Niederman R (1992) Alteration of cytoplasmic Ca2+ in resting and stimulated human neutrophils by short-chain carboxylic acids at neutral pH. Infect Immun 60:5307–5311
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L (2017) Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551:585
Smolinska S, Jutel M, Crameri R, O’Mahony L (2014) Histamine and gut mucosal immune regulation. Allergy 69:273–281
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278:11312–11319
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohloolyy M, Glickman JN, Garrett WS (2013) The microbial metabolites, short chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Chang HK (2015) Short chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8:80
Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M (2008) Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9:194–202
Xu L, Kitani A, Stuelten C, Mcgrady G, Fuss I, Strober W (2010) Positive and negative transcriptional regulation of the Foxp3 gene is mediated by TGF-Î2 signal transducer smad3 access and binding to enhancer I. Immunity 33:313
Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, Sakaguchi S, Takeda K (2012) Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. J Immunol 189:2869–2878
Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S (2007) Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity 27:145–159
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277:32–48
Kim M, Qie Y, Park J, Chang HK (2016) Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20:202–214
Peterson DA, Mcnulty NP, Guruge JL, Gordon JI (2007) IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2:328–339
Agace WW, McCoy KD (2017) Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity 46:532–548
Pott J, Hornef M (2012) Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep 13:684–698
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345
Willemsen LEM, Koetsier MA, van Deventer SJH, van Tol EAF (2003) Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 52:1442–1447
Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156:1045–1059
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139
Liu L, Guo X, Rao JN, Zou T, Xiao L, Yu T, Timmons JA, Turner DJ, Wang JY (2009) Polyamines regulate E-cadherin transcription through c-Myc modulating intestinal epithelial barrier function. Am J Physiol-Cell Ph 296:C801–C810
Sonnenberg GF, Artis D (2012) Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37:601–610
Walker JA, Barlow JL, McKenzie ANJ (2013) Innate lymphoid cells–how did we miss them? Nature reviews. Immunology 13:75–87
Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva E, Wilhelm C, Veldhoen M (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147:629–640
Lee JS, Cella M, Mcdonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD (2012) AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13:144–151
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2010) Regulation and functions of IL-10 family cytokines in inflammation and diseases. Annu Rev Immunol 29:71–109
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231–234
Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W (2011) IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol 12:941–948
Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu Y, Tumanov AV (2010) Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 32:403–413
Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L (2012) The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36:92–104
Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O (2011) Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol 127:701–721
Mahony LO, Akdis M, Akdis CA (2011) Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol 128:1153–1162
Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V (2010) Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 285:27601–27608
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngombru C, Blanchard C, Junt T, Nicod LP, Harris NL (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159
Liu L, Li L, Min J, Wang J, Wu H, Zeng Y, Chen S, Chu Z (2012) Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol 277:66–73
Burris TP, Busby SA, Griffin PR (2012) Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity. Chem Biol 19:51–59
Fiorucci Stefano, Mencarelli Andrea, Palladino Giuseppe, Cipriani Sabrina (2009) Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci 30:570–580
Yu D (2011) The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 54:1421–1432
Brestoff JR, Artis D (2013) Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 14:676
Giordano D, Magaletti DM, Clark EA, Beavo JA (2003) Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN + (CD209) intermediate cell and impair differentiation into dendritic cells. J Immunol 171:6421–6430
Cassatella MA (1995) The production of cytokines by polymorphonuclear neutrophils. Immunol Today 16:21–26
Oliver JM (1978) Cell biology of leukocyte abnormalities–membrane and cytoskeletal function in normal and defective cells. A review. Am J Pathol 93:221
Wagner JG, Roth RA (2000) Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 52:349
Le PE, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van DJ (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278:25481–25489
Wong JM, De SR, Kendall CW, Emam A, Jenkins DJ (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243
Maslowski KM, Vieira AT, Aylwin N, Jan K, Frederic S, Di Y, Schilter HC, Rolph MS, Fabienne M, David A (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286
Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A (2009) G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol 136:240
Fialkow L, Wang Y, Downey GP (2007) Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42:153–164
Irani K, Goldschmidtclermont PJ (2000) Ras, superoxide and signal transduction. Biochem Pharmacol 55:47–79
Kanai T, Kawamura T, Dohi T, Makita S, Nemoto Y, Totsuka T, Watanabe M (2006) TH1/TH2-mediated colitis induced by adoptive transfer of CD4+ CD45RBhigh T lymphocytes into nude mice. Inflamm Bowel Dis 12:89–99
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12:661–672
Brown EM, Kenny DJ, Xavier RJ (2019) Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu Rev Immunol 37:599–624
Shi Y, Mu L (2017) An expanding stage for commensal microbes in host immune regulation. Cell Mol Immunol 14:339
Tanoue T, Atarashi K, Honda K (2016) Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 16:295
Arpaia N, Campbell C, Fan X, Dikiy S, Van JDV, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451
Coombes JL, Siddiqui KR, Arancibiacárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F (2007) A functionally specialized population of mucosal CD103 + DCs induces Foxp3 + regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764
Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256–260
Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204:1775–1785
Chen WJ, Jin W, Hardegen N, Lei K, Li L, Marinos N, Mcgrady G, Wahl SM (2003) Conversion of peripheral CD4 + CD25 − naive T cells to CD4 + CD25 + regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198:1875–1886
Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Sitahar M, Santo JPD, Eberl G (2008) In vivo equilibrium of proinflammatory IL-17 + and regulatory IL-10 + Foxp3 + RORγt + T cells. J Exp Med 205:1381–1393
Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriaurouthiau V, Marques R, Dulauroy S, Fedoseeva M (2015) Mucosal immunology. The microbiota regulates type 2 immunity through RORγt? T cells. Science 349:989–993
Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH (2007) Vitamin A metabolites induce gut-homing FoxP3 + regulatory T cells. J Immunol 179:3724–3733
Huehn J, Siegmund K, Lehmann JCU, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK (2004) Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4 + regulatory T cells. J Exp Med 199:303–313
Martens JH, Barg H, Warren M, Jahn D (2002) Microbial production of vitamin B12. Appl Microbiol Biot 58:275–285
Hill MJ (1997) Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prevent 6(Suppl 1):S43–S45
Strozzi GP, Mogna L (2008) Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J Clin Gastroenterol 42(Suppl 3 Pt 2):S179–S184
Pompei A, Cordisco L, Amaretti A, Zanoni S, Matteuzzi D, Rossi M (2007) Folate production by bifidobacteria as a potential probiotic property. Appl Environ Microb 73:179
Kleerebezem M, Vaughan EE (2009) Probiotic and gut lactobacilli and bifidobacteria: molecular approaches to study diversity and activity. Annu Rev Microbiol 63:269–290
Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, Saitoh T, Kurabayshi H, Naruse T (1999) Immunomodulation by vitamin B12: augmentation of CD8 + T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol 116:28–32
Honda K, Dan RL (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535:75
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446
Ferstl R, Akdis CA, O’Mahony L (2012) Histamine regulation of innate and adaptive immunity. Front Biosci 17:40
Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW (2009) Mast cells down-regulate CD4 + CD25 + T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol 183:3014–3022
Kudoh K, Shimizu JM, Takita T, Kanke Y, Innami S (1998) Effect of indigestible saccharides on B lymphocyte response of intestinal mucosa and cecal fermentation in rats. J Nutr Sci Vitaminol 44:103–112
Lim BO, Yamada K, Nonaka M, Kuramoto Y, Hung P, Sugano M (1997) Dietary fibers modulate indices of intestinal immune function in rats. The Journal of nutrition 127:663–667
Caromaldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR (2014) Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 192:3626–3636
Niccolai E, Boem F, Russo E, Amedei A (2019) The gut-brain axis in the neuropsychological disease model of obesity: a classical movie revised by the emerging director “microbiome”. Nutrients 11:156
Bliss ES, Whiteside E (2018) The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol 2018:9
Mayer EA (2011) Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci 12:453
Rezzi S, Ramadan Z, Martin FJ, Fay LB, van Bladeren P, Lindon JC, Nicholson JK, Kochhar S (2007) Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J Proteome Res 6:4469–4477
Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS (2012) Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis 18:2301–2309
Amatya N, Garg AV, Gaffen SL (2017) IL-17 Signaling: the Yin and the Yang. Trends Immunol 38:310–322
Haase S, Haghikia A, Wilck N, Müller DN, Linker RA (2018) Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 154:230–238
Borre YE, Keeffe GWO, Clarke G, Stanton C, Dinan TG, Cryan JF (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20:509–518
Torresfuentes C, Schellekens H, Dinan TG, Cryan JF (2017) The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol 2:747–756
Braniste V, Alasmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263
Frost G, Sleeth ML, Sahuriarisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir MK, Zhang S (2014) The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611
Rothhammer V, Borucki DM, Tjon E, Takenaka MC, Chao C, Ardurafabregat A, De Lima KA, Gutierrezvazquez C, Hewson P, Staszewski O (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557:724–728
Nicolas GR, Chang PV (2019) Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol Sci 40:430–445
Amedei A, Boem F (2018) I’ve gut A feeling: microbiota impacting the conceptual and experimental perspectives of personalized medicine. Int J Mol Sci 19:3756
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31420103908), the National Key R&D Program of China (no. 2017YFD0500506) and the European Union’s H2020 project Feed-a-Gene (no. 633531). We thank Dr. L. Johnston and Dr. C. Levesque for revising the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, G., Huang, S., Wang, Y. et al. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 76, 3917–3937 (2019). https://doi.org/10.1007/s00018-019-03190-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03190-6