Abstract
Intracellular traffic amongst organelles represents a key feature for eukaryotes and is orchestrated principally by members of Rab family, the largest within Ras superfamily. Given that variations in Rab repertoire have been fundamental in animal diversification, we provided the most exhaustive survey regarding the Rab toolkit of chordates. Our findings reveal the existence of 42 metazoan conserved subfamilies exhibiting a univocal intron/exon structure preserved from cnidarians to vertebrates. Since the current view does not capture the Rab complexity, we propose a new Rab family classification in three distinct monophyletic clades. The Rab complement of chordates shows a dramatic diversification due to genome duplications and independent gene duplications and losses with sharp differences amongst cephalochordates, tunicates and gnathostome vertebrates. Strikingly, the analysis of the domain architecture of this family highlighted the existence of chimeric calcium-binding Rabs, which are animal novelties characterized by a complex evolutionary history in gnathostomes and whose role in cellular metabolism is obscure. This work provides novel insights in the knowledge of Rab family: our hypothesis is that chordates represent a hotspot of Rab variability, with many events of gene gains and losses impacting intracellular traffic capabilities. Our results help to elucidate the role of Rab members in the transport amongst endomembranes and shed light on intracellular traffic routes in vertebrates. Then, since the predominant role of Rabs in the molecular communication between different cellular districts, this study paves to way to comprehend inherited or acquired human disorders provoked by dysfunctions in Rab genes.





Similar content being viewed by others
References
Szathmary E, Smith JM (1995) The major evolutionary transitions. Nature 374(6519):227–232
Touchot N, Chardin P, Tavitian A (1987) Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci USA 84(23):8210–8214
Diekmann Y, Seixas E, Gouw M, Tavares-Cadete F, Seabra MC, Pereira-Leal JB (2011) Thousands of rab GTPases for the cell biologist. PLoS Comput Biol 7(10):e1002217
Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10(8):513–525
Lee MT, Mishra A, Lambright DG (2009) Structural mechanisms for regulation of membrane traffic by rab GTPases. Traffic 10(10):1377–1389
Park HH (2013) Structural basis of membrane trafficking by Rab family small G protein. Int J Mol Sci 14(5):8912–8923
Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116(2):153–166
Sztul E, Lupashin V (2009) Role of vesicle tethering factors in the ER-Golgi membrane traffic. FEBS Lett 583(23):3770–3783
Goud B, Liu S, Storrie B (2018) Rab proteins as major determinants of the Golgi complex structure. Small GTPases 9(1–2):66–75
Barr F, Lambright DG (2010) Rab GEFs and GAPs. Curr Opin Cell Biol 22(4):461–470
Alexandrov K, Horiuchi H, Steele-Mortimer O, Seabra MC, Zerial M (1994) Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J 13(22):5262–5273
Shi CH, Zhang SY, Yang ZH, Yang J, Shang DD, Mao CY, Liu H, Hou HM, Shi MM, Wu J et al (2016) A novel RAB39B gene mutation in X-linked juvenile parkinsonism with basal ganglia calcification. Mov Disord 31(12):1905–1909
Banworth MJ, Li G (2018) Consequences of Rab GTPase dysfunction in genetic or acquired human diseases. Small GTPases 9(1–2):158–181
Nishimura N, Van Huyen Pham T, Hartomo TB, Lee MJ, Hasegawa D, Takeda H, Kawasaki K, Kosaka Y, Yamamoto T, Morikawa S et al (2011) Rab15 expression correlates with retinoic acid-induced differentiation of neuroblastoma cells. Oncol Rep 26(1):145–151
Pham TV, Hartomo TB, Lee MJ, Hasegawa D, Ishida T, Kawasaki K, Kosaka Y, Yamamoto T, Morikawa S, Yamamoto N et al (2012) Rab15 alternative splicing is altered in spheres of neuroblastoma cells. Oncol Rep 27(6):2045–2049
Hendrix A, De Wever O (2013) Rab27 GTPases distribute extracellular nanomaps for invasive growth and metastasis: implications for prognosis and treatment. Int J Mol Sci 14(5):9883–9892
Qin X, Wang J, Wang X, Liu F, Jiang B, Zhang Y (2017) Targeting Rabs as a novel therapeutic strategy for cancer therapy. Drug Discov Today 22(8):1139–1147
Klopper TH, Kienle N, Fasshauer D, Munro S (2012) Untangling the evolution of Rab G proteins: implications of a comprehensive genomic analysis. BMC Biol 10:71
Stenmark H (2012) The Rabs: a family at the root of metazoan evolution. BMC Biol 10:68
Dunst S, Kazimiers T, von Zadow F, Jambor H, Sagner A, Brankatschk B, Mahmoud A, Spannl S, Tomancak P, Eaton S et al (2015) Endogenously tagged rab proteins: a resource to study membrane trafficking in Drosophila. Dev Cell 33(3):351–365
Cavalier-Smith T (2009) Predation and eukaryote cell origins: a coevolutionary perspective. Int J Biochem Cell Biol 41(2):307–322
Elias M, Brighouse A, Gabernet-Castello C, Field MC, Dacks JB (2012) Sculpting the endomembrane system in deep time: high resolution phylogenetics of Rab GTPases. J Cell Sci 125(Pt 10):2500–2508
Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S et al (2007) Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315(5809):207–212
Saito-Nakano Y, Nakahara T, Nakano K, Nozaki T, Numata O (2010) Marked amplification and diversification of products of ras genes from rat brain, Rab GTPases, in the ciliates Tetrahymena thermophila and Paramecium tetraurelia. J Eukaryot Microbiol 57(5):389–399
Lal K, Field MC, Carlton JM, Warwicker J, Hirt RP (2005) Identification of a very large Rab GTPase family in the parasitic protozoan Trichomonas vaginalis. Mol Biochem Parasitol 143(2):226–235
Pereira-Leal JB, Seabra MC (2001) Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 313(4):889–901
Rutherford S, Moore I (2002) The Arabidopsis Rab GTPase family: another enigma variation. Curr Opin Plant Biol 5(6):518–528
Brighouse A, Dacks JB, Field MC (2010) Rab protein evolution and the history of the eukaryotic endomembrane system. Cell Mol Life Sci 67(20):3449–3465
Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu JK et al (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453(7198):1064–1071
Pennati R, Ficetola GF, Brunetti R, Caicci F, Gasparini F, Griggio F, Sato A, Stach T, Kaul-Strehlow S, Gissi C et al (2015) Morphological differences between larvae of the Ciona intestinalis species complex: hints for a valid taxonomic definition of distinct species. PLoS One 10(5):e0122879
Berna L, Alvarez-Valin F (2014) Evolutionary genomics of fast evolving tunicates. Genome Biol Evol 6(7):1724–1738
Rojas AM, Fuentes G, Rausell A, Valencia A (2012) The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 196(2):189–201
Huet D, Blisnick T, Perrot S, Bastin P (2014) The GTPase IFT27 is involved in both anterograde and retrograde intraflagellar transport. Elife 3:e02419
Gallegos ME, Balakrishnan S, Chandramouli P, Arora S, Azameera A, Babushekar A, Bargoma E, Bokhari A, Chava SK, Das P et al (2012) The C. elegans rab family: identification, classification and toolkit construction. PLoS One 7(11):e49387
Iida H, Noda M, Kaneko T, Doiguchi M, Mori T (2005) Identification of rab12 as a vesicle-associated small GTPase highly expressed in Sertoli cells of rat testis. Mol Reprod Dev 71(2):178–185
Piloto S, Schilling TF (2010) Ovo1 links Wnt signaling with N-cadherin localization during neural crest migration. Development 137(12):1981–1990
Matsui T, Fukuda M (2011) Small GTPase Rab12 regulates transferrin receptor degradation: implications for a novel membrane trafficking pathway from recycling endosomes to lysosomes. Cell Logist 1(4):155–158
Uno T, Ozakiya Y, Furutani M, Sakamoto K, Uno Y, Kajiwara H, Kanamaru K, Mizoguchi A (2018) Functional characterization of insect-specific RabX6 of Bombyx mori. Histochem Cell Biol 151:187–198
Cañestro C, Albalat R (2012) Transposon diversity is higher in amphioxus than in vertebrates: functional and evolutionary inferences. Brief Funct Genom 11(2):131–141
Paps J, Holland PW, Shimeld SM (2012) A genome-wide view of transcription factor gene diversity in chordate evolution: less gene loss in amphioxus? Brief Funct Genom 11(2):177–186
Somorjai IML, Martí-Solans J, Diaz-Gracia M, Nishida H, Imai KS, Escriva H, Cañestro C, Albalat R (2018) Wnt evolution and function shuffling in liberal and conservative chordate genomes. Genome Biol 19(1):98
Holland LZ, Albalat R, Azumi K, Benito-Gutierrez E, Blow MJ, Bronner-Fraser M, Brunet F, Butts T, Candiani S, Dishaw LJ et al (2008) The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res 18(7):1100–1111
Abi-Rached L, Gilles A, Shiina T, Pontarotti P, Inoko H (2002) Evidence of en bloc duplication in vertebrate genomes. Nat Genet 31(1):100–105
Dehal P, Boore JL (2005) Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3(10):e314
Catchen JM, Conery JS, Postlethwait JH (2009) Automated identification of conserved synteny after whole-genome duplication. Genome Res 19(8):1497–1505
D’Aniello S, Irimia M, Maeso I, Pascual-Anaya J, Jimenez-Delgado S, Bertrand S, Garcia-Fernandez J (2008) Gene expansion and retention leads to a diverse tyrosine kinase superfamily in amphioxus. Mol Biol Evol 25(9):1841–1854
Irimia M, Roy SW (2008) Spliceosomal introns as tools for genomic and evolutionary analysis. Nucleic Acids Res 36(5):1703–1712
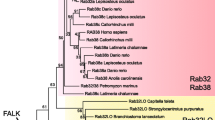
Coppola U, Annona G, D’Aniello S, Ristoratore F (2016) Rab32 and Rab38 genes in chordate pigmentation: an evolutionary perspective. BMC Evol Biol 16:26
Melchior F, Paschal B, Evans J, Gerace L (1993) Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol 123(6 Pt 2):1649–1659
Coppola U, Caccavale F, Scelzo M, Holland ND, Ristoratore F, D’Aniello S (2018) Ran GTPase, an eukaryotic gene novelty, is involved in amphioxus mitosis. PLoS One 13(10):e0196930
Tan R, Wang W, Wang S, Wang Z, Sun L, He W, Fan R, Zhou Y, Xu X, Hong W et al (2013) Small GTPase Rab40c associates with lipid droplets and modulates the biogenesis of lipid droplets. PLoS One 8(4):e63213
Yatsu A, Shimada H, Ohbayashi N, Fukuda M (2015) Rab40C is a novel Varp-binding protein that promotes proteasomal degradation of Varp in melanocytes. Biol Open 4(3):267–275
Nakayama S, Moncrief ND, Kretsinger RH (1992) Evolution of EF-hand calcium-modulated proteins. II. Domains of several subfamilies have diverse evolutionary histories. J Mol Evol 34(5):416–448
Shintani M, Tada M, Kobayashi T, Kajiho H, Kontani K, Katada T (2007) Characterization of Rab45/RASEF containing EF-hand domain and a coiled-coil motif as a self-associating GTPase. Biochem Biophys Res Commun 357(3):661–667
Srikanth S, Jung HJ, Kim KD, Souda P, Whitelegge J, Gwack Y (2010) A novel EF-hand protein, CRACR55A, is a cytosolic Ca2 + sensor that stabilizes CRAC channels in T cells. Nat Cell Biol 12(5):436–446
Albalat R, Cañestro C (2016) Evolution by gene loss. Nat Rev Genet 17(7):379–391
Denoeud F, Henriet S, Mungpakdee S, Aury JM, Da Silva C, Brinkmann H, Mikhaleva J, Olsen LC, Jubin C, Cañestro C et al (2010) Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science 330(6009):1381–1385
Martí-Solans J, Belyaeva OV, Torres-Aguila NP, Kedishvili NY, Albalat R, Cañestro C (2016) Coelimination and survival in gene network evolution: dismantling the RA-signaling in a chordate. Mol Biol Evol 33(9):2401–2416
Flood PR, Afzelius BA (1978) The spermatozoon of Oikopleura dioica Fol (Larvacea, Tunicata). Cell Tissue Res 191(1):27–37
Onuma TA, Isobe M, Nishida H (2017) Internal and external morphology of adults of the appendicularian, Oikopleura dioica: an SEM study. Cell Tissue Res 367(2):213–227
Hoegg S, Brinkmann H, Taylor JS, Meyer A (2004) Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol 59(2):190–203
Kuraku S, Meyer A (2009) The evolution and maintenance of Hox gene clusters in vertebrates and the teleost-specific genome duplication. Int J Dev Biol 53(5–6):765–773
Lien S, Koop BF, Sandve SR, Miller JR, Kent MP, Nome T, Hvidsten TR, Leong JS, Minkley DR, Zimin A et al (2016) The Atlantic salmon genome provides insights into rediploidization. Nature 533(7602):200–205
Cañestro C, Albalat R, Irimia M, Garcia-Fernandez J (2013) Impact of gene gains, losses and duplication modes on the origin and diversification of vertebrates. Semin Cell Dev Biol 24(2):83–94
Woichansky I, Beretta CA, Berns N, Riechmann V (2016) Three mechanisms control E-cadherin localization to the zonula adherens. Nat Commun 7:10834
Chen L, Hu J, Yun Y, Wang T (2010) Rab36 regulates the spatial distribution of late endosomes and lysosomes through a similar mechanism to Rab34. Mol Membr Biol 27(1):23–30
Olkkonen VM, Ikonen E (2006) When intracellular logistics fails–genetic defects in membrane trafficking. J Cell Sci 119(Pt 24):5031–5045
Oshita H, Nishino R, Takano A, Fujitomo T, Aragaki M, Kato T, Akiyama H, Tsuchiya E, Kohno N, Nakamura Y et al (2013) RASEF is a novel diagnostic biomarker and a therapeutic target for lung cancer. Mol Cancer Res 11(8):937–951
Kaplon J, Homig-Holzel C, Gao L, Meissl K, Verdegaal EM, van der Burg SH, van Doorn R, Peeper DS (2014) Near-genomewide RNAi screening for regulators of BRAF(V600E)-induced senescence identifies RASEF, a gene epigenetically silenced in melanoma. Pigment Cell Melanoma Res 27(4):640–652
Maat W, Beiboer SH, Jager MJ, Luyten GP, Gruis NA, van der Velden PA (2008) Epigenetic regulation identifies RASEF as a tumor-suppressor gene in uveal melanoma. Invest Ophthalmol Vis Sci 49(4):1291–1298
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform 5:113
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948
Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55(4):539–552
Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O (2011) Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60(5):685–699
Marletaz F, Firbas PN, Maeso I, Tena JJ, Bogdanovic O, Perry M, Wyatt CDR, de la Calle-Mustienes E, Bertrand S, Burguera D et al (2018) Amphioxus functional genomics and the origins of vertebrate gene regulation. Nature 564:64–70
Acknowledgements
The authors are grateful to the Branchiostoma lanceolatum Genome Consortium that provided access to the European amphioxus genome [76]. We would like to thank Dr. Eva Jimenez-Guri for her critical reading of the manuscript, and three anonymous reviewers for their comments that helped to improve the manuscript. R.A. was supported by BIO2015-67358-C2-1-P grant from Ministerio de Economía y Competitividad (Spain) and by Grant SGR2017-1665 from Generalitat de Catalunya. U.C. was supported by a OU-SZN PhD fellowship.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
– Raw phylogenetic trees (A, B) including Rab names. (PDF 5642 kb)
Rights and permissions
About this article
Cite this article
Coppola, U., Ristoratore, F., Albalat, R. et al. The evolutionary landscape of the Rab family in chordates. Cell. Mol. Life Sci. 76, 4117–4130 (2019). https://doi.org/10.1007/s00018-019-03103-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03103-7