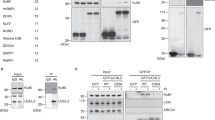
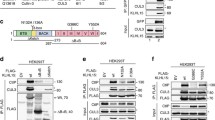
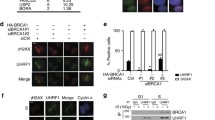
Abstract
The ability of cells to repair DNA double-strand breaks (DSBs) is important for maintaining genome stability and eliminating oncogenic DNA lesions. Two distinct and complementary pathways, non-homologous end joining (NHEJ) and homologous recombination (HR), are employed by mammalian cells to repair DNA DSBs. Each pathway is tightly controlled in response to increased DSBs. The Ku heterodimer has been shown to play a regulatory role in NHEJ repair. Ku80 ubiquitination contributes to the selection of a DSB repair pathway by causing the removal of Ku heterodimers from DSB sites. However, whether Ku80 deubiquitination also plays a role in regulating DSB repair is unknown. To address this question, we performed a comprehensive study of the deubiquitinase specific for Ku80, and our study showed that the deubiquitinase OTUD5 serves as an important regulator of NHEJ repair by increasing the stability of Ku80. Further studies revealed that OTUD5 depletion impaired NHEJ repair, and hence reduced overall DSB repair. Furthermore, OTUD5-depleted cells displayed excess end resection; as a result, HR repair was facilitated by OTUD5 depletion during the S/G2 phase. In summary, our study demonstrates that OTUD5 is a specific deubiquitinase for Ku80 and establishes OTUD5 as an important and positive regulator of NHEJ repair.






Similar content being viewed by others
References
Khanna KK, Jackson SP (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27:247–254. https://doi.org/10.1038/85798
Streffer C (2010) Strong association between cancer and genomic instability. Radiat Environ Biophys 49:125–131. https://doi.org/10.1007/s00411-009-0258-4
Lieber MR (2008) The mechanism of human nonhomologous DNA end joining. J Biol Chem 283:1–5. https://doi.org/10.1074/jbc.R700039200
Lieber MR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79:181–211. https://doi.org/10.1146/annurev.biochem.052308.093131
Mazon G, Mimitou EP, Symington LS (2010) SnapShot: homologous recombination in DNA double-strand break repair. Cell 142(646):e641. https://doi.org/10.1016/j.cell.2010.08.006
San Filippo J, Sung P, Klein H (2008) Mechanism of eukaryotic homologous recombination. Annu Rev Biochem 77:229–257. https://doi.org/10.1146/annurev.biochem.77.061306.125255
Chiruvella KK, Liang Z, Wilson TE (2013) Repair of double-strand breaks by end joining. Cold Spring Harb Perspect Biol 5:a012757. https://doi.org/10.1101/cshperspect.a012757
Karanam K, Kafri R, Loewer A, Lahav G (2012) Quantitative live cell imaging reveals a gradual shift between DNA repair mechanisms and a maximal use of HR in mid S phase. Mol Cell 47:320–329. https://doi.org/10.1016/j.molcel.2012.05.052
Shim EY et al (2010) Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J 29:3370–3380. https://doi.org/10.1038/emboj.2010.219
Postow L (2011) Destroying the ring: freeing DNA from Ku with ubiquitin. FEBS Lett 585:2876–2882. https://doi.org/10.1016/j.febslet.2011.05.046
Postow L et al (2008) Ku80 removal from DNA through double strand break-induced ubiquitylation. J Cell Biol 182:467–479. https://doi.org/10.1083/jcb.200802146
Ismail IH et al (2015) The RNF138 E3 ligase displaces Ku to promote DNA end resection and regulate DNA repair pathway choice. Nat Cell Biol 17:1446–1457. https://doi.org/10.1038/ncb3259
Feng L, Chen J (2012) The E3 ligase RNF8 regulates KU80 removal and NHEJ repair. Nat Struct Mol Biol 19:201–206. https://doi.org/10.1038/nsmb.2211
Ishida N et al (2017) Ubiquitylation of Ku80 by RNF126 promotes completion of nonhomologous end joining-mediated DNA repair. Mol Cell Biol 37:e00347-16. https://doi.org/10.1128/mcb.00347-16
Nishi R et al (2018) The deubiquitylating enzyme UCHL3 regulates Ku80 retention at sites of DNA damage. Sci Rep 8:17891. https://doi.org/10.1038/s41598-018-36235-0
Abdul Rehman SA et al (2016) MINDY-1 Is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol Cell 63:146–155. https://doi.org/10.1016/j.molcel.2016.05.009
Nijman SM et al (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786. https://doi.org/10.1016/j.cell.2005.11.007
Bekker-Jensen S, Mailand N (2015) RNF138 joins the HR team. Nat Cell Biol 17:1375–1377. https://doi.org/10.1038/ncb3262
Huang OW et al (2012) Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat Struct Mol Biol 19:171–175. https://doi.org/10.1038/nsmb.2206
Mevissen TE et al (2013) OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154:169–184. https://doi.org/10.1016/j.cell.2013.05.046
Britton S, Coates J, Jackson SP (2013) A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol 202:579–595. https://doi.org/10.1083/jcb.201303073
Pierce AJ, Johnson RD, Thompson LH, Jasin M (1999) XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev 13:2633–2638
Ogiwara H et al (2011) Histone acetylation by CBP and p300 at double-strand break sites facilitates SWI/SNF chromatin remodeling and the recruitment of non-homologous end joining factors. Oncogene 30:2135–2146. https://doi.org/10.1038/onc.2010.592
Chapman JR, Taylor MR, Boulton SJ (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47:497–510. https://doi.org/10.1016/j.molcel.2012.07.029
Symington LS, Gautier J (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45:247–271. https://doi.org/10.1146/annurev-genet-110410-132435
Ma HT, Poon RY (2017) Synchronization of HeLa cells. Methods Mol Biol 1524:189–201. https://doi.org/10.1007/978-1-4939-6603-5_12
Gupta R et al (2018) DNA repair network analysis reveals shieldin as a key regulator of NHEJ and PARP inhibitor sensitivity. Cell 173:972–988. https://doi.org/10.1016/j.cell.2018.03.050
Pierce AJ, Hu P, Han M, Ellis N, Jasin M (2001) Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev 15:3237–3242. https://doi.org/10.1101/gad.946401
Grob P et al (2012) Electron microscopy visualization of DNA-protein complexes formed by Ku and DNA ligase IV. DNA Repair (Amst) 11:74–81. https://doi.org/10.1016/j.dnarep.2011.10.023
Wu D, Topper LM, Wilson TE (2008) Recruitment and dissociation of nonhomologous end joining proteins at a DNA double-strand break in Saccharomyces cerevisiae. Genetics 178:1237–1249. https://doi.org/10.1534/genetics.107.083535
Niu H, Raynard S, Sung P (2009) Multiplicity of DNA end resection machineries in chromosome break repair. Genes Dev 23:1481–1486. https://doi.org/10.1101/gad.1824209
Postow L, Funabiki H (2013) An SCF complex containing Fbxl12 mediates DNA damage-induced Ku80 ubiquitylation. Cell Cycle 12:587–595. https://doi.org/10.4161/cc.23408
de Vivo A et al (2019) The OTUD5-UBR5 complex regulates FACT-mediated transcription at damaged chromatin. Nucleic Acids Res 47:729–746. https://doi.org/10.1093/nar/gky1219
Difilippantonio MJ et al (2000) DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature 404:510–514. https://doi.org/10.1038/35006670
Lim DS et al (2000) Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol Cell Biol 20:3772–3780
Wei S et al (2012) Ku80 functions as a tumor suppressor in hepatocellular carcinoma by inducing S-phase arrest through a p53-dependent pathway. Carcinogenesis 33:538–547. https://doi.org/10.1093/carcin/bgr319
Shan J, Zhao W, Gu W (2009) Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 36:469–476. https://doi.org/10.1016/j.molcel.2009.10.018
Yu M et al (2016) USP11 is a negative regulator to gammaH2AX ubiquitylation by RNF8/RNF168. J Biol Chem 291:959–967. https://doi.org/10.1074/jbc.M114.624478
Rodrigue A et al (2006) Interplay between human DNA repair proteins at a unique double-strand break in vivo. EMBO J 25:222–231. https://doi.org/10.1038/sj.emboj.7600914
Acknowledgements
We are grateful to Dr. Jiadong Wang for his suggestion and help. We thank Qihua He for her suggestion and help, and Center of Medical and Health Analysis, Peking University, for confocal microscopy. We appreciate the ALENABIO (Xi’an, China) Company (http://www.alenabio.com) for the pathological micro-tissues (Cat. No. BC03119a). We deeply appreciate help from Ning Kon for editing our manuscript.
Funding
Wenhui Zhao was supported by the National Natural Science Foundation of China (Grant No. 85141044).
Author information
Authors and Affiliations
Contributions
FL and WZ, conceived and designed the study that led to the submission, acquired data, and interpreted the results; FL performed the experiment; QS, KL, HH, QH, ZC, YM, and FT participated in the revision of the manuscript; ZM and TT approved the final version; and WZ was the corresponding author. The authors declare that they have no conflicts of interest associated with the contents of this article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Availability of data and material
All of the data and material in this paper are available upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, F., Sun, Q., Liu, K. et al. The deubiquitinase OTUD5 regulates Ku80 stability and non-homologous end joining. Cell. Mol. Life Sci. 76, 3861–3873 (2019). https://doi.org/10.1007/s00018-019-03094-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03094-5