Abstract
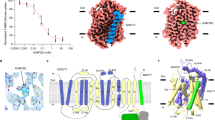
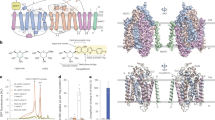
Monocarboxylate transporter 8 (MCT8) mediates thyroid hormone (TH) transport across the plasma membrane in many cell types. In order to better understand its mechanism, we have generated three new MCT8 homology models based on sugar transporters XylE in the intracellular opened (PDB ID: 4aj4) and the extracellular partly occluded (PDB ID: 4gby) conformations as well as FucP (PDB ID: 3o7q) and GLUT3 (PDB ID: 4zwc) in the fully extracellular opened conformation. T3-docking studies from both sides revealed interactions with His192, His415, Arg445 and Asp498 as previously identified. Selected mutations revealed further transport-sensitive positions mainly at the discontinuous transmembrane helices TMH7 and 10. Lys418 is potentially involved in neutralising the charge of the TH substrate because it can be replaced by charged, but not by uncharged, amino acids. The side chain of Thr503 was hypothesised to stabilise a helix break at TMH10 that undergoes a prominent local shift during the transport cycle. A T503V mutation accordingly affected transport. The aromatic Tyr419, the polar Ser313 and Ser314 as well as the charged Glu422 and Glu423 lining the transport channel have been studied. Based on related sugar transporters, we suggest an alternating access mechanism for MCT8 involving a series of amino acid positions previously and newly identified as critical for transport.










Similar content being viewed by others
Abbreviations
- AHDS:
-
Allan–Herndon–Dudley syndrome
- FucP:
-
E. coli fucose/H+ symporter
- GlpT:
-
Glycerol-3-phosphate transporter
- GLUT3:
-
Glucose transporter 3
- T3 :
-
3,3′,5-Triiodothyronie
- T4 :
-
3,3′,5,5′-Tetraiodothyronine
- TH:
-
Thyroid hormone
- TM:
-
Transmembrane
- TMH:
-
Transmembrane helix
- XylE:
-
E. coli d-xylose:H+ symporter
References
Abe T, Kakyo M, Sakagami H et al (1998) Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem 273:22395–22401. doi:10.1074/jbc.273.35.22395
Sugiyama D, Kusuhara H, Taniguchi H et al (2003) Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood–brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495. doi:10.1074/jbc.M306933200
Tohyama K, Kusuhara H, Sugiyama Y (2004) Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood–brain barrier. Endocrinology 145:4384–4391. doi:10.1210/en.2004-0058
Friesema ECH, Ganguly S, Abdalla A et al (2003) Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135. doi:10.1074/jbc.M300909200
Friesema ECH, Jansen J, Jachtenberg J-W et al (2008) Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22:1357–1369. doi:10.1210/me.2007-0112
Friesema EC, Docter R, Moerings EP et al (2001) Thyroid hormone transport by the heterodimeric human system l amino acid transporter. Endocrinology 142:4339–4348
Zevenbergen C, Meima ME, Lima de Souza EC et al (2015) Transport of iodothyronines by human l-type amino acid transporters. Endocrinology 156:4345–4355. doi:10.1210/en.2015-1140
Kinne A, Wittner M, Wirth EK et al (2015) Involvement of the l-type amino acid transporter Lat2 in the transport of 3,3′-diiodothyronine across the plasma membrane. Eur Thyroid J 4:42–50. doi:10.1159/000381542
Schweizer U, Johannes J, Bayer D, Braun D (2014) Structure and function of thyroid hormone plasma membrane transporters. Eur Thyroid J 3:143–153. doi:10.1159/000367858
Friesema ECH, Grueters PA, Biebermann H et al (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437. doi:10.1016/S0140-6736(04)17226-7
Dumitrescu AM, Liao XH, Best TB et al (2004) A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175
Schwartz CE, May MM, Carpenter NJ et al (2005) Allan–Herndon–Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77:41–53
Dumitrescu AM, Liao XH, Weiss RE et al (2006) Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (Mct) 8-deficient mice. Endocrinology 147:4036–4043. doi:10.1210/en.2006-0390
Trajkovic M, Visser T, Mittag J (2007) Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635. doi:10.1172/JCI28253.A
Wirth EK, Roth S, Blechschmidt C et al (2009) Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan–Herndon–Dudley syndrome. J Neurosci 29:9439–9449
Mayerl S, Müller J, Bauer R et al (2014) Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 124:1987–1999. doi:10.1172/JCI70324
Roberts LM, Woodford K, Zhou M et al (2008) Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood–brain barrier. Endocrinology 149:6251–6261. doi:10.1210/en.2008-0378
Shi Y (2013) Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys 42:51–72. doi:10.1146/annurev-biophys-083012-130429
Huang Y, Lemieux MJ, Song J et al (2003) Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301(80):616–620. doi:10.1126/science.1087619
Kinne A, Kleinau G, Hoefig CS et al (2010) Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J Biol Chem 285:28054–28063. doi:10.1074/jbc.M110.129577
Groeneweg S, Friesema ECH, Kersseboom S et al (2014) The role of Arg445 and Asp498 in the human thyroid hormone transporter MCT8. Endocrinology 155:618–626. doi:10.1210/en.2013-1521
Ye L, Li YL, Mellström K et al (2003) Thyroid receptor ligands. 1. Agonist ligands selective for the thyroid receptor β1. J Med Chem 46:1580–1588. doi:10.1021/jm021080f
Nascimento AS, Dias SMG, Nunes FM et al (2006) Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J Mol Biol 360:586–598. doi:10.1016/j.jmb.2006.05.008
Braun D, Lelios I, Krause G, Schweizer U (2013) Histidines in potential substrate recognition sites affect thyroid hormone transport by monocarboxylate transporter 8 (MCT8). Endocrinology 154:2553–2561. doi:10.1210/en.2012-2197
Kleinau G, Schweizer U, Kinne A et al (2011) Insights into molecular properties of the human monocarboxylate transporter 8 by combining functional with structural information. Thyroid Res 4 (Suppl 1):S4. doi:10.1186/1756-6614-4-S1-S4
Groeneweg S, Lima de Souza EC, Visser WE et al (2013) Importance of His192 in the human thyroid hormone transporter MCT8 for substrate recognition. Endocrinology 154:2525–2532. doi:10.1210/en.2012-2225
Lima de Souza EC, Groeneweg S, Visser WE et al (2013) Importance of cysteine residues in the thyroid hormone transporter MCT8. Endocrinology 154:1948–1955. doi:10.1210/en.2012-2101
Quistgaard EM, Löw C, Moberg P et al (2013) Structural basis for substrate transport in the GLUT-homology family of monosaccharide transporters. Nat Struct Mol Biol 20:766–768. doi:10.1038/nsmb.2569
Sun L, Zeng X, Yan C et al (2012) Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490:361–366. doi:10.1038/nature11524
Medlock AE, Dailey TA, Ross TA et al (2007) A pi-helix switch selective for porphyrin deprotonation and product release in human ferrochelatase. J Mol Biol 373:1006–1016. doi:10.1016/j.jmb.2007.08.040
Deng D, Sun P, Yan C et al (2015) Molecular basis of ligand recognition and transport by glucose transporters. Nature 526:391–396. doi:10.1038/nature14655
Dang S, Sun L, Huang Y et al (2010) Structure of a fucose transporter in an outward-open conformation. Nature 467:734–738. doi:10.1038/nature09406
Schrödinger LLC (2016) The PyMOL molecular graphics system, Version 1.8.0.1
Braun D, Kim TD, le Coutre P et al (2012) Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J Clin Endocrinol Metab 97:E100–E105. doi:10.1210/jc.2011-1837
Braun D, Schweizer U (2015) Efficient activation of pathogenic ∆Phe501 mutation in monocarboxylate transporter 8 by chemical and pharmacological chaperones. Endocrinology 156:4720–4730. doi:10.1210/en.2015-1393
Johannes J, Braun D, Kinne A et al (2016) Few amino acid exchanges expand the substrate spectrum of monocarboxylate transporter 10. Mol Endocrinol. doi:10.1210/me.2016-1037
Kinne A, Roth S, Biebermann H et al (2009) Surface translocation and tri-iodothyronine uptake of mutant MCT8 proteins are cell type-dependent. J Mol Endocrinol 43:263–271. doi:10.1677/JME-09-0043
Fu J, Refetoff S, Dumitrescu AM, Weiss RE (2014) OR29-3: whole-exome sequencing identified a novel MCT8 gene mutation in a child with mild cognitive, motor and behavior abnormalities. Endocr Rev 35:OR29-OR33. doi:10.1210/endo-meetings.2014.THPTA.1.OR29-3
Nomura N, Verdon G, Kang HJ et al (2015) Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 526:397–401. doi:10.1038/nature14909
Screpanti E, Hunte C (2007) Discontinuous membrane helices in transport proteins and their correlation with function. J Struct Biol 159:261–267. doi:10.1016/j.jsb.2007.01.011
Jansen J, Friesema ECH, Kester MHA et al (2008) Genotype-phenotype relationship in patients with mutations in thyroid hormone transporter MCT8. Endocrinology 149:2184–2190. doi:10.1210/en.2007-1475
Anik A, Kersseboom S, Demir K et al (2014) Psychomotor retardation caused by a defective thyroid hormone transporter: report of two families with different MCT8 mutations. Horm Res Paediatr 82:261–271. doi:10.1159/000365191
Visser WE, Jansen J, Friesema ECH et al (2009) Novel pathogenic mechanism suggested by ex vivo analysis of MCT8 (SLC16A2) mutations. Hum Mutat 30:29–38. doi:10.1002/humu.20808
Fischer J, Kleinau G, Müller A et al (2015) Modulation of monocarboxylate transporter 8 oligomerization by specific pathogenic mutations. J Mol Endocrinol 54:39–50. doi:10.1530/JME-14-0272
Raymond L, Whibley A, Price S et al (2008) Raised T3 levels and mutations in MCT8(SLC16A2) cause X-linked cerebral palsy and mental retardation. Eur J Hum Genet 16:60
Friesema ECH, Visser WE, Visser TJ (2010) Genetics and phenomics of thyroid hormone transport by MCT8. Mol Cell Endocrinol 322:107–113. doi:10.1016/j.mce.2010.01.016
Ono E, Ariga M, Oshima S et al (2016) Three novel mutations of the MCT8 (SLC16A2) gene: individual and temporal variations of endocrinological and radiological features. Clin Pediatr Endocrinol 25:23–35. doi:10.1297/cpe.25.23
Ugrasbul F, Ardinger HH (2009) A patient presenting with central hypothyroidism, developmental delay and poor head control. Should we be checking T3 levels? Horm Res 72(Suppl 3):458–459. doi:10.1159/000239668
Acknowledgements
The authors thank Simone Arndt and Tobias Lindenberg for excellent technical assistance, Catherine L. Worth for critically reading the paper before submission and acknowledge funding by Deutsche Forschungsgemeinschaft DFG THYROID TRANS ACT KR1273/5-1 (GK), Schw914/3-1 (US) and the Sherman family (US).
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Protze and D. Braun, as well as U. Schweizer and G. Krause contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Protze, J., Braun, D., Hinz, K.M. et al. Membrane-traversing mechanism of thyroid hormone transport by monocarboxylate transporter 8. Cell. Mol. Life Sci. 74, 2299–2318 (2017). https://doi.org/10.1007/s00018-017-2461-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2461-9