Abstract
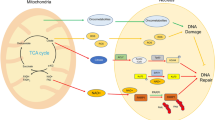
To maintain genome stability, cells have evolved various DNA repair pathways to deal with oxidative DNA damage. DNA damage response (DDR) pathways, including ATM-Chk2 and ATR-Chk1 checkpoints, are also activated in oxidative stress to coordinate DNA repair, cell cycle progression, transcription, apoptosis, and senescence. Several studies demonstrate that DDR pathways can regulate DNA repair pathways. On the other hand, accumulating evidence suggests that DNA repair pathways may modulate DDR pathway activation as well. In this review, we summarize our current understanding of how various DNA repair and DDR pathways are activated in response to oxidative DNA damage primarily from studies in eukaryotes. In particular, we analyze the functional interplay between DNA repair and DDR pathways in oxidative stress. A better understanding of cellular response to oxidative stress may provide novel avenues of treating human diseases, such as cancer and neurodegenerative disorders.







Similar content being viewed by others
Abbreviations
- 9-1-1 complex:
-
Rad9-Rad1-Hus1
- AP:
-
Apurinic/apyrimidinic
- APE1:
-
AP endonuclease 1
- APE2:
-
AP endonuclease 2
- A-T:
-
Ataxia-telangiectasia
- ATM:
-
A-T mutated
- ATR:
-
ATM- and Rad3-related
- BER:
-
Base excision repair
- Chk1:
-
Checkpoint kinase 1
- Chk2:
-
Checkpoint kinase 2
- DDR:
-
DNA damage response
- DSB:
-
Double-strand break
- GG-NER:
-
Global genome NER
- HR:
-
Homologous recombination
- Ku complex:
-
Ku70/Ku80
- MCM:
-
Minichromosome maintenance
- MMR:
-
Mismatch repair
- MRN complex:
-
Mre11-Rad50-Nbs1
- NER:
-
Nucleotide excision repair
- NHEJ:
-
Non-homologous end joining
- PCNA:
-
Proliferating cell nuclear antigen
- ROS:
-
Reactive oxygen species
- RPA:
-
Replication protein A
- SSB:
-
Single-strand break
- SSBR:
-
SSB repair
- ssDNA:
-
Single-stranded DNA
- TC-NER:
-
Transcription-coupled NER
- TDP1:
-
Tyrosyl-DNA phosphodiesterase 1
- γ-H2AX:
-
H2AX phosphorylation at Serine 139
References
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Betteridge DJ (2000) What is oxidative stress? Metabolism 49(2 Suppl 1):3–8
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8(9–10):1865–1879. doi:10.1089/ars.2006.8.1865
de M Bandeira S, da Fonseca LJ, da SGG, Rabelo LA, Goulart MO, Vasconcelos SM (2013) Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci 14(2):3265–3284. doi:10.3390/ijms14023265
Agnez-Lima LF, Melo JT, Silva AE, Oliveira AH, Timoteo AR, Lima-Bessa KM, Martinez GR, Medeiros MH, Di Mascio P, Galhardo RS, Menck CF (2012) DNA damage by singlet oxygen and cellular protective mechanisms. Mutat Res 751(1):15–28. doi:10.1016/j.mrrev.2011.12.005
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90(17):7915–7922
Berquist BR, Wilson DM 3rd (2012) Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett 327(1–2):61–72. doi:10.1016/j.canlet.2012.02.001
Riley PA (1994) Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65(1):27–33
Dizdaroglu M (2012) Oxidatively induced DNA damage: mechanisms, repair and disease. Cancer Lett 327(1–2):26–47. doi:10.1016/j.canlet.2012.01.016
Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB (2004) Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol 14(3):259–266. doi:10.1016/j.semradonc.2004.04.001
Barzilai A, Rotman G, Shiloh Y (2002) ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 1(1):3–25
Barzilai A, Yamamoto K (2004) DNA damage responses to oxidative stress. DNA Repair (Amst) 3(8–9):1109–1115. doi:10.1016/j.dnarep.2004.03.002
Gafter-Gvili A, Zingerman B, Rozen-Zvi B, Ori Y, Green H, Lubin I, Malachi T, Gafter U, Herman-Edelstein M (2013) Oxidative stress-induced DNA damage and repair in human peripheral blood mononuclear cells: protective role of hemoglobin. PLoS One 8(7):e68341. doi:10.1371/journal.pone.0068341
Gutowski M, Kowalczyk S (2013) A study of free radical chemistry: their role and pathophysiological significance. Acta Biochim Pol 60(1):1–16
Rubattu S, Mennuni S, Testa M, Mennuni M, Pierelli G, Pagliaro B, Gabriele E, Coluccia R, Autore C, Volpe M (2013) Pathogenesis of chronic cardiorenal syndrome: is there a role for oxidative stress? Int J Mol Sci 14(11):23011–23032. doi:10.3390/ijms141123011
Cadet J, Loft S, Olinski R, Evans MD, Bialkowski K, Richard Wagner J, Dedon PC, Moller P, Greenberg MM, Cooke MS (2012) Biologically relevant oxidants and terminology, classification and nomenclature of oxidatively generated damage to nucleobases and 2-deoxyribose in nucleic acids. Free Radic Res 46(4):367–381. doi:10.3109/10715762.2012.659248
Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362:709–715
Friedberg EC (2003) DNA damage and repair. Nature 421(6921):436–440. doi:10.1038/nature01408
Ciccia A, Elledge SJ (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40(2):179–204. doi:10.1016/j.molcel.2010.09.019
Hoeijmakers JH (2009) DNA damage, aging, and cancer. N Engl J Med 361(15):1475–1485. doi:10.1056/NEJMra0804615
Cadet J, Ravanat JL, TavernaPorro M, Menoni H, Angelov D (2012) Oxidatively generated complex DNA damage: tandem and clustered lesions. Cancer Lett 327(1–2):5–15. doi:10.1016/j.canlet.2012.04.005
Neeley WL, Essigmann JM (2006) Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol 19(4):491–505. doi:10.1021/tx0600043
Litwin I, Bocer T, Dziadkowiec D, Wysocki R (2013) Oxidative stress and replication-independent DNA breakage induced by arsenic in Saccharomyces cerevisiae. PLoS Genet 9(7):e1003640. doi:10.1371/journal.pgen.1003640
Kessel M, Liu SX, Xu A, Santella R, Hei TK (2002) Arsenic induces oxidative DNA damage in mammalian cells. Mol Cell Biochem 234–235(1–2):301–308
Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B (2011) Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther 10(12):2224–2233. doi:10.1158/1535-7163.MCT-11-0534
Bresgen N, Karlhuber G, Krizbai I, Bauer H, Bauer HC, Eckl PM (2003) Oxidative stress in cultured cerebral endothelial cells induces chromosomal aberrations, micronuclei, and apoptosis. J Neurosci Res 72(3):327–333. doi:10.1002/jnr.10582
Cadet J, Douki T, Ravanat JL (2011) Measurement of oxidatively generated base damage in cellular DNA. Mutat Res 711(1–2):3–12. doi:10.1016/j.mrfmmm.2011.02.004
Lee SF, Pervaiz S (2011) Assessment of oxidative stress-induced DNA damage by immunoflourescent analysis of 8-oxodG. Methods Cell Biol 103:99–113. doi:10.1016/B978-0-12-385493-3.00005-X
Andersson M, Stenqvist P, Hellman B (2007) Interindividual differences in initial DNA repair capacity when evaluating H2O2-induced DNA damage in extended-term cultures of human lymphocytes using the comet assay. Cell Biol Toxicol 23(6):401–411. doi:10.1007/s10565-007-9002-5
Glei M, Hovhannisyan G, Pool-Zobel BL (2009) Use of Comet-FISH in the study of DNA damage and repair: review. Mutat Res 681(1):33–43. doi:10.1016/j.mrrev.2008.01.006
Chen BP, Li M, Asaithamby A (2012) New insights into the roles of ATM and DNA-PKcs in the cellular response to oxidative stress. Cancer Lett 327(1–2):103–110. doi:10.1016/j.canlet.2011.12.004
Cimprich KA, Cortez D (2008) ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 9(8):616–627. doi:10.1038/nrm2450
Wallace SS, Murphy DL, Sweasy JB (2012) Base excision repair and cancer. Cancer Lett 327(1–2):73–89. doi:10.1016/j.canlet.2011.12.038
Raetz AG, Xie Y, Kundu S, Brinkmeyer MK, Chang C, David SS (2012) Cancer-associated variants and a common polymorphism of MUTYH exhibit reduced repair of oxidative DNA damage using a GFP-based assay in mammalian cells. Carcinogenesis 33(11):2301–2309. doi:10.1093/carcin/bgs270
Xiao X, Melton DW, Gourley C (2013) Mismatch repair deficiency in ovarian cancer—molecular characteristics and clinical implications. Gynecol Oncol 132(2):506–512. doi:10.1016/j.ygyno.2013.12.003
Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA (2008) DNA repair pathways as targets for cancer therapy. Nat Rev Cancer 8(3):193–204. doi:10.1038/nrc2342
Bouwman P, Jonkers J (2012) The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer 12(9):587–598. doi:10.1038/nrc3342
Hegde ML, Mantha AK, Hazra TK, Bhakat KK, Mitra S, Szczesny B (2012) Oxidative genome damage and its repair: implications in aging and neurodegenerative diseases. Mech Ageing Dev 133(4):157–168. doi:10.1016/j.mad.2012.01.005
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461(7267):1071–1078. doi:10.1038/nature08467
Marechal A, Zou L (2013) DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5(9):a012716. doi:10.1101/cshperspect.a012716
Branzei D, Foiani M (2010) Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol 11(3):208–219. doi:10.1038/nrm2852
Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15(17):2177–2196. doi:10.1101/gad.914401
Harrison JC, Haber JE (2006) Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet 40:209–235. doi:10.1146/annurev.genet.40.051206.105231
Finn K, Lowndes NF, Grenon M (2012) Eukaryotic DNA damage checkpoint activation in response to double-strand breaks. Cell Mol Life Sci 69(9):1447–1473. doi:10.1007/s00018-011-0875-3
Karagiannis TC, El-Osta A (2004) Double-strand breaks: signaling pathways and repair mechanisms. Cell Mol Life Sci 61(17):2137–2147. doi:10.1007/s00018-004-4174-0
Khanna KK, Jackson SP (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27(3):247–254. doi:10.1038/85798
van Gent DC, Hoeijmakers JH, Kanaar R (2001) Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet 2(3):196–206. doi:10.1038/35056049
Lavin MF (2008) Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9(10):759–769. doi:10.1038/nrm2514
Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NG, Taylor AM, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y (1995) A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268(5218):1749–1753
Bakkenist CJ, Kastan MB (2004) Initiating cellular stress responses. Cell 118(1):9–17. doi:10.1016/j.cell.2004.06.023
Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421(6922):499–506. doi:10.1038/nature01368
Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y (2003) Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 22(20):5612–5621. doi:10.1093/emboj/cdg541
van den Bosch M, Bree RT, Lowndes NF (2003) The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep 4(9):844–849. doi:10.1038/sj.embor.embor925
Costanzo V, Paull T, Gottesman M, Gautier J (2004) Mre11 assembles linear DNA fragments into DNA damage signaling complexes. PLoS Biol 2(5):E110. doi:10.1371/journal.pbio.0020110
Lee JH, Paull TT (2005) ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308(5721):551–554. doi:10.1126/science.1108297
Lee JH, Paull TT (2007) Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 26(56):7741–7748. doi:10.1038/sj.onc.1210872
Lee JH, Paull TT (2004) Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304(5667):93–96. doi:10.1126/science.1091496
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273(10):5858–5868
Franco S, Gostissa M, Zha S, Lombard DB, Murphy MM, Zarrin AA, Yan C, Tepsuporn S, Morales JC, Adams MM, Lou Z, Bassing CH, Manis JP, Chen J, Carpenter PB, Alt FW (2006) H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell 21(2):201–214. doi:10.1016/j.molcel.2006.01.005
Schultz LB, Chehab NH, Malikzay A, Halazonetis TD (2000) p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol 151(7):1381–1390
Goldberg M, Stucki M, Falck J, D’Amours D, Rahman D, Pappin D, Bartek J, Jackson SP (2003) MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature 421(6926):952–956. doi:10.1038/nature01445
Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP (2005) MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123(7):1213–1226. doi:10.1016/j.cell.2005.09.038
Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, Alt FW, Chen J (2006) MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell 21(2):187–200. doi:10.1016/j.molcel.2005.11.025
Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J (2007) RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131(5):901–914. doi:10.1016/j.cell.2007.09.041
Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP (2009) Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462(7275):935–939. doi:10.1038/nature08657
Yin Y, Seifert A, Chua JS, Maure JF, Golebiowski F, Hay RT (2012) SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev 26(11):1196–1208. doi:10.1101/gad.189274.112
Galanty Y, Belotserkovskaya R, Coates J, Jackson SP (2012) RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev 26(11):1179–1195. doi:10.1101/gad.188284.112
Okuno Y, Nakamura-Ishizu A, Otsu K, Suda T, Kubota Y (2012) Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med 18(8):1208–1216. doi:10.1038/nm.2846
Singh S, Englander EW (2012) Nuclear depletion of apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1) is an indicator of energy disruption in neurons. Free Radic Biol Med 53(9):1782–1790. doi:10.1016/j.freeradbiomed.2012.07.025
Chen K, Albano A, Ho A, Keaney JF Jr (2003) Activation of p53 by oxidative stress involves platelet-derived growth factor-beta receptor-mediated ataxia telangiectasia mutated (ATM) kinase activation. J Biol Chem 278(41):39527–39533. doi:10.1074/jbc.M304423200
Hammond EM, Dorie MJ, Giaccia AJ (2003) ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem 278(14):12207–12213. doi:10.1074/jbc.M212360200
Cui J, Liu J, Wu S, Wang Y, Shen H, Xing L, Wang J, Yan X, Zhang X (2013) Oxidative DNA damage is involved in ochratoxin A-induced G2 arrest through ataxia telangiectasia-mutated (ATM) pathways in human gastric epithelium GES-1 cells in vitro. Arch Toxicol 87(10):1829–1840. doi:10.1007/s00204-013-1043-3
Rotman G, Shiloh Y (1997) Ataxia-telangiectasia: is ATM a sensor of oxidative damage and stress? BioEssays 19(10):911–917. doi:10.1002/bies.950191011
Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT (2010) ATM activation by oxidative stress. Science 330(6003):517–521. doi:10.1126/science.1192912
Ambrose M, Gatti RA (2013) Pathogenesis of ataxia-telangiectasia: the next generation of ATM functions. Blood 121(20):4036–4045. doi:10.1182/blood-2012-09-456897
Bhatti S, Kozlov S, Farooqi AA, Naqi A, Lavin M, Khanna KK (2011) ATM protein kinase: the linchpin of cellular defenses to stress. Cell Mol Life Sci 68(18):2977–3006. doi:10.1007/s00018-011-0683-9
Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL (2010) ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci USA 107(9):4153–4158. doi:10.1073/pnas.0913860107
Kanu N, Penicud K, Hristova M, Wong B, Irvine E, Plattner F, Raivich G, Behrens A (2010) The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain. J Biol Chem 285(49):38534–38542. doi:10.1074/jbc.M110.145896
Harper JW, Elledge SJ (2007) The DNA damage response: ten years after. Mol Cell 28(5):739–745. doi:10.1016/j.molcel.2007.11.015
Cimprich KA, Shin TB, Keith CT, Schreiber SL (1996) cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc Natl Acad Sci USA 93(7):2850–2855
Brown EJ, Baltimore D (2000) ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev 14(4):397–402
O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33(4):497–501. doi:10.1038/ng1129
Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316(5828):1160–1166. doi:10.1126/science.1140321
Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA, Gygi SP, Livingstone M, Zhang H, Polakiewicz RD, Comb MJ (2007) Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci USA 104(50):19855–19860. doi:10.1073/pnas.0707579104
Guo Z, Kumagai A, Wang SX, Dunphy WG (2000) Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev 14(21):2745–2756
Willis J, Patel Y, Lentz BL, Yan S (2013) APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Proc Natl Acad Sci USA 110(26):10592–10597. doi:10.1073/pnas.1301445110
Zhao H, Piwnica-Worms H (2001) ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol 21(13):4129–4139. doi:10.1128/MCB.21.13.4129-4139.2001
Yan S, Willis J (2013) WD40-repeat protein WDR18 collaborates with TopBP1 to facilitate DNA damage checkpoint signaling. Biochem Biophys Res Commun 431(3):466–471. doi:10.1016/j.bbrc.2012.12.144
Willis J, Destephanis D, Patel Y, Gowda V, Yan S (2012) Study of the DNA damage checkpoint using Xenopus egg extracts. J Vis Exp 69:e4449. doi:10.3791/4449
Bai L, Michael WM, Yan S (2014) Importin beta-dependent nuclear import of TopBP1 in ATR-Chk1 checkpoint in Xenopus egg extracts. Cell Signal 26(5):857–867. doi:10.1016/j.cellsig.2014.01.006
Sanchez Y (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277(5331):1497–1501. doi:10.1126/science.277.5331.1497
Boddy MN (1998) Replication checkpoint enforced by kinases Cds1 and Chk1. Science 280(5365):909–912. doi:10.1126/science.280.5365.909
Zhang Y, Hunter T (2013) Roles of Chk1 in cell biology and cancer therapy. Int J Cancer 134(5):1013–1023. doi:10.1002/ijc.28226
Nam EA, Cortez D (2011) ATR signalling: more than meeting at the fork. Biochem J 436(3):527–536. doi:10.1042/BJ20102162
Bartek J, Lukas C, Lukas J (2004) Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 5(10):792–804. doi:10.1038/nrm1493
MacDougall CA, Byun TS, Van C, Yee MC, Cimprich KA (2007) The structural determinants of checkpoint activation. Genes Dev 21(8):898–903. doi:10.1101/gad.1522607
Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA (2005) Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev 19(9):1040–1052. doi:10.1101/gad.1301205
You Z, Shi LZ, Zhu Q, Wu P, Zhang YW, Basilio A, Tonnu N, Verma IM, Berns MW, Hunter T (2009) CtIP links DNA double-strand break sensing to resection. Mol Cell 36(6):954–969. doi:10.1016/j.molcel.2009.12.002
Giannattasio M, Follonier C, Tourriere H, Puddu F, Lazzaro F, Pasero P, Lopes M, Plevani P, Muzi-Falconi M (2010) Exo1 competes with repair synthesis, converts NER intermediates to long ssDNA gaps, and promotes checkpoint activation. Mol Cell 40(1):50–62. doi:10.1016/j.molcel.2010.09.004
Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300(5625):1542–1548. doi:10.1126/science.1083430
Cortez D, Guntuku S, Qin J, Elledge SJ (2001) ATR and ATRIP: partners in checkpoint signaling. Science 294(5547):1713–1716. doi:10.1126/science.1065521
Ellison V, Stillman B (2003) Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol 1(2):E33. doi:10.1371/journal.pbio.0000033
Parrilla-Castellar ER, Arlander SJ, Karnitz L (2004) Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair 3(8–9):1009–1014. doi:10.1016/j.dnarep.2004.03.032
Bermudez VP, Lindsey-Boltz LA, Cesare AJ, Maniwa Y, Griffith JD, Hurwitz J, Sancar A (2003) Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc Natl Acad Sci USA 100(4):1633–1638. doi:10.1073/pnas.0437927100
Majka J, Binz SK, Wold MS, Burgers PM (2006) Replication protein A directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J Biol Chem 281(38):27855–27861. doi:10.1074/jbc.M605176200
Kondo T, Wakayama T, Naiki T, Matsumoto K, Sugimoto K (2001) Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science 294(5543):867–870. doi:10.1126/science.1063827
Melo JA, Cohen J, Toczyski DP (2001) Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev 15(21):2809–2821. doi:10.1101/gad.903501
Kanoh Y, Tamai K, Shirahige K (2006) Different requirements for the association of ATR-ATRIP and 9-1-1 to the stalled replication forks. Gene 377:88–95. doi:10.1016/j.gene.2006.03.019
Garcia V, Furuya K, Carr AM (2005) Identification and functional analysis of TopBP1 and its homologs. DNA Repair (Amst) 4(11):1227–1239. doi:10.1016/j.dnarep.2005.04.001
Sokka M, Parkkinen S, Pospiech H, Syvaoja JE (2010) Function of TopBP1 in genome stability. Subcell Biochem 50:119–141. doi:10.1007/978-90-481-3471-7_7
Van Hatten RA, Tutter AV, Holway AH, Khederian AM, Walter JC, Michael WM (2002) The Xenopus Xmus101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J Cell Biol 159(4):541–547. doi:10.1083/jcb.200207090
Hashimoto Y, Takisawa H (2003) Xenopus Cut5 is essential for a CDK-dependent process in the initiation of DNA replication. EMBO J 22(10):2526–2535. doi:10.1093/emboj/cdg238
Kumagai A, Lee J, Yoo HY, Dunphy WG (2006) TopBP1 activates the ATR-ATRIP complex. Cell 124(5):943–955. doi:10.1016/j.cell.2005.12.041
Mordes DA, Glick GG, Zhao R, Cortez D (2008) TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes Dev 22(11):1478–1489. doi:10.1101/gad.1666208
St Onge RP, Besley BD, Pelley JL, Davey S (2003) A role for the phosphorylation of hRad9 in checkpoint signaling. J Biol Chem 278(29):26620–26628. doi:10.1074/jbc.M303134200
Greer DA, Besley BDA, Kennedy KB, Davey S (2003) hRad9 rapidly binds DNA containing double-strand breaks and is required for damage-dependent Topoisomerase II binding protein 1 focus formation. Cancer Res 63:4829–4835
Furuya K, Poitelea M, Guo L, Caspari T, Carr AM (2004) Chk1 activation requires Rad9 S/TQ-site phosphorylation to promote association with C-terminal BRCT domains of Rad4TOPBP1. Genes Dev 18(10):1154–1164. doi:10.1101/gad.291104
Delacroix S, Wagner JM, Kobayashi M, Yamamoto K, Karnitz LM (2007) The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev 21(12):1472–1477. doi:10.1101/gad.1547007
Yan S, Lindsay HD, Michael WM (2006) Direct requirement for Xmus101 in ATR-mediated phosphorylation of Claspin bound Chk1 during checkpoint signaling. J Cell Biol 173(2):181–186. doi:10.1083/jcb.200601076
Taricani L, Wang TS (2006) Rad4TopBP1, a scaffold protein, plays separate roles in DNA damage and replication checkpoints and DNA replication. Mol Biol Cell 17(8):3456–3468. doi:10.1091/mbc.E06-01-0056
Parrilla-Castellar ER, Karnitz LM (2003) Cut5 is required for the binding of Atr and DNA polymerase alpha to genotoxin-damaged chromatin. J Biol Chem 278(46):45507–45511. doi:10.1074/jbc.C300418200
Yan S, Michael WM (2009) TopBP1 and DNA polymerase-alpha directly recruit the 9-1-1 complex to stalled DNA replication forks. J Cell Biol 184(6):793–804. doi:10.1083/jcb.200810185
Gong Z, Kim JE, Leung CC, Glover JN, Chen J (2010) BACH1/FANCJ acts with TopBP1 and participates early in DNA replication checkpoint control. Mol Cell 37(3):438–446. doi:10.1016/j.molcel.2010.01.002
Wang J, Gong Z, Chen J (2011) MDC1 collaborates with TopBP1 in DNA replication checkpoint control. J Cell Biol 193(2):267–273. doi:10.1083/jcb.201010026
Duursma AM, Driscoll R, Elias JE, Cimprich KA (2013) A role for the MRN complex in ATR activation via TOPBP1 recruitment. Mol Cell 50(1):116–122. doi:10.1016/j.molcel.2013.03.006
Lee J, Dunphy WG (2013) The Mre11-Rad50-Nbs1 (MRN) complex has a specific role in the activation of Chk1 in response to stalled replication forks. Mol Biol Cell 24(9):1343–1353. doi:10.1091/mbc.E13-01-0025
Kumagai A, Dunphy WG (2000) Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell 6(4):839–849. doi:10.1016/S1097-2765(05)00092-4
Chini CC, Chen J (2003) Human claspin is required for replication checkpoint control. J Biol Chem 278(32):30057–30062. doi:10.1074/jbc.M301136200
Lin SY, Li K, Stewart GS, Elledge SJ (2004) Human Claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc Natl Acad Sci USA 101(17):6484–6489. doi:10.1073/pnas.0401847101
Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A (2005) Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol 25(8):3109–3116. doi:10.1128/MCB.25.8.3109-3116.2005
Unsal-Kacmaz K, Chastain PD, Qu PP, Minoo P, Cordeiro-Stone M, Sancar A, Kaufmann WK (2007) The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol 27(8):3131–3142. doi:10.1128/MCB.02190-06
Errico A, Costanzo V, Hunt T (2007) Tipin is required for stalled replication forks to resume DNA replication after removal of aphidicolin in Xenopus egg extracts. Proc Natl Acad Sci USA 104(38):14929–14934. doi:10.1073/pnas.0706347104
Errico A, Costanzo V (2012) Mechanisms of replication fork protection: a safeguard for genome stability. Crit Rev Biochem Mol Biol 47(3):222–235. doi:10.3109/10409238.2012.655374
Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19(2):238–245. doi:10.1016/j.ceb.2007.02.009
Yoo HY, Kumagai A, Shevchenko A, Dunphy WG (2004) Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell 117(5):575–588
Kulkarni A, Das KC (2008) Differential roles of ATR and ATM in p53, Chk1, and histone H2AX phosphorylation in response to hyperoxia: ATR-dependent ATM activation. Am J Physiol Lung Cell Mol Physiol 294(5):L998–L1006. doi:10.1152/ajplung.00004.2008
Yang Y, Durando M, Smith-Roe SL, Sproul C, Greenwalt AM, Kaufmann W, Oh S, Hendrickson EA, Vaziri C (2013) Cell cycle stage-specific roles of Rad18 in tolerance and repair of oxidative DNA damage. Nucleic Acids Res 41(4):2296–2312. doi:10.1093/nar/gks1325
Jang SH, Lim JW, Morio T, Kim H (2012) Lycopene inhibits Helicobacter pylori-induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic Biol Med 52(3):607–615. doi:10.1016/j.freeradbiomed.2011.11.010
Cuadrado M, Martinez-Pastor B, Murga M, Toledo LI, Gutierrez-Martinez P, Lopez E, Fernandez-Capetillo O (2006) ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J Exp Med 203(2):297–303. doi:10.1084/jem.20051923
Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP (2006) ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8(1):37–45. doi:10.1038/ncb1337
Shiotani B, Zou L (2009) Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell 33(5):547–558. doi:10.1016/j.molcel.2009.01.024
Gobbini E, Cesena D, Galbiati A, Lockhart A, Longhese MP (2013) Interplays between ATM/Tel1 and ATR/Mec1 in sensing and signaling DNA double-strand breaks. DNA Repair (Amst) 12(10):791–799. doi:10.1016/j.dnarep.2013.07.009
Caporali S, Falcinelli S, Starace G, Russo MT, Bonmassar E, Jiricny J, D’Atri S (2004) DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol 66(3):478–491. doi:10.1124/mol.66.3
Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, O’Driscoll M, Jeggo PA (2006) ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J 25(24):5775–5782. doi:10.1038/sj.emboj.7601446
Yajima H, Lee KJ, Zhang S, Kobayashi J, Chen BP (2009) DNA double-strand break formation upon UV-induced replication stress activates ATM and DNA-PKcs kinases. J Mol Biol 385(3):800–810. doi:10.1016/j.jmb.2008.11.036
Bauer M, Goldstein M, Christmann M, Becker H, Heylmann D, Kaina B (2011) Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress. Proc Natl Acad Sci USA 108(52):21105–21110. doi:10.1073/pnas.1111919109
Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, Awasthi YC (2013) 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J Biol Chem 288(28):20532–20546. doi:10.1074/jbc.M113.467662
Ward IM, Chen J (2001) Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 276(51):47759–47762. doi:10.1074/jbc.C100569200
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276(45):42462–42467. doi:10.1074/jbc.C100466200
Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408(6811):433–439
Curtin NJ (2012) DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 12(12):801–817. doi:10.1038/nrc3399
Slupphaug G (2003) The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res 531(1–2):231–251. doi:10.1016/j.mrfmmm.2003.06.002
Meira LB, Burgis NE, Samson LD (2005) Base excision repair. Adv Exp Med Biol 570:125–173. doi:10.1007/1-4020-3764-3_5
Cao W (2013) Endonuclease V: an unusual enzyme for repair of DNA deamination. Cell Mol Life Sci 70(17):3145–3156. doi:10.1007/s00018-012-1222-z
Svilar D, Goellner EM, Almeida KH, Sobol RW (2011) Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid Redox Signal 14(12):2491–2507. doi:10.1089/ars.2010.3466
Krokan HE, Bjoras M (2013) Base excision repair. Cold Spring Harb Perspect Biol 5(4):a012583. doi:10.1101/cshperspect.a012583
Caldecott KW (2008) Single-strand break repair and genetic disease. Nat Rev Genet 9(8):619–631. doi:10.1038/nrg2380
Melis JP, van Steeg H, Luijten M (2013) Oxidative DNA damage and nucleotide excision repair. Antioxid Redox Signal 18(18):2409–2419. doi:10.1089/ars.2012.5036
Batty DP, Wood RD (2000) Damage recognition in nucleotide excision repair of DNA. Gene 241(2):193–204
Guo C, Tang TS, Friedberg EC (2010) SnapShot: nucleotide excision repair. Cell 140(5):754–754.e1. doi:10.1016/j.cell.2010.02.033
Brierley DJ, Martin SA (2013) Oxidative stress and the DNA mismatch repair pathway. Antioxid Redox Signal 18(18):2420–2428. doi:10.1089/ars.2012.4994
Jiricny J (2013) Postreplicative mismatch repair. Cold Spring Harb Perspect Biol 5(4):a012633. doi:10.1101/cshperspect.a012633
San Filippo J, Sung P, Klein H (2008) Mechanism of eukaryotic homologous recombination. Annu Rev Biochem 77:229–257. doi:10.1146/annurev.biochem.77.061306.125255
Symington LS, Gautier J (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45:247–271. doi:10.1146/annurev-genet-110410-132435
Lieber MR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79:181–211. doi:10.1146/annurev.biochem.052308.093131
Sirbu BM, Cortez D (2013) DNA damage response: three levels of DNA repair regulation. Cold Spring Harb Perspect Biol 5(8):a012724. doi:10.1101/cshperspect.a012724
Helt CE, Wang W, Keng PC, Bambara RA (2005) Evidence that DNA damage detection machinery participates in DNA repair. Cell Cycle 4(4):529–532
Chou WC, Wang HC, Wong FH, Ding SL, Wu PE, Shieh SY, Shen CY (2008) Chk2-dependent phosphorylation of XRCC1 in the DNA damage response promotes base excision repair. EMBO J 27(23):3140–3150. doi:10.1038/emboj.2008.229
Meagher M, Lightowlers RN (2014) The role of TDP1 and APTX in mitochondrial DNA repair. Biochimie 100:121–124. doi:10.1016/j.biochi.2013.10.011
Das BB, Antony S, Gupta S, Dexheimer TS, Redon CE, Garfield S, Shiloh Y, Pommier Y (2009) Optimal function of the DNA repair enzyme TDP1 requires its phosphorylation by ATM and/or DNA-PK. EMBO J 28(23):3667–3680. doi:10.1038/emboj.2009.302
Povirk LF (2012) Processing of damaged DNA ends for double-strand break repair in mammalian cells. ISRN Mol Biol 2012:345805. doi:10.5402/2012/345805
Qi Y, Schoene NW, Lartey FM, Cheng WH (2010) Selenium compounds activate ATM-dependent DNA damage response via the mismatch repair protein hMLH1 in colorectal cancer cells. J Biol Chem 285(43):33010–33017. doi:10.1074/jbc.M110.137406
Romeo F, Falbo L, Di Sanzo M, Misaggi R, Faniello MC, Viglietto G, Cuda G, Costanzo F, Quaresima B (2011) BRCA1 is required for hMLH1 stabilization following doxorubicin-induced DNA damage. Int J Biochem Cell Biol 43(12):1754–1763. doi:10.1016/j.biocel.2011.08.011
Lee JH, Kim KH, Morio T, Kim H (2006) Ataxia-telangiectasia-mutated-dependent activation of Ku in human fibroblasts exposed to hydrogen peroxide. Ann NY Acad Sci 1091:76–82. doi:10.1196/annals.1378.056
Kuhne C, Tjornhammar ML, Pongor S, Banks L, Simoncsits A (2003) Repair of a minimal DNA double-strand break by NHEJ requires DNA-PKcs and is controlled by the ATM/ATR checkpoint. Nucleic Acids Res 31(24):7227–7237
Di Virgilio M, Ying CY, Gautier J (2009) PIKK-dependent phosphorylation of Mre11 induces MRN complex inactivation by disassembly from chromatin. DNA Repair (Amst) 8(11):1311–1320. doi:10.1016/j.dnarep.2009.07.006
Toueille M, El-Andaloussi N, Frouin I, Freire R, Funk D, Shevelev I, Friedrich-Heineken E, Villani G, Hottiger MO, Hubscher U (2004) The human Rad9/Rad1/Hus1 damage sensor clamp interacts with DNA polymerase beta and increases its DNA substrate utilisation efficiency: implications for DNA repair. Nucleic Acids Res 32(11):3316–3324. doi:10.1093/nar/gkh652
Wang W, Brandt P, Rossi ML, Lindsey-Boltz L, Podust V, Fanning E, Sancar A, Bambara RA (2004) The human Rad9-Rad1-Hus1 checkpoint complex stimulates flap endonuclease 1. Proc Natl Acad Sci USA 101(48):16762–16767. doi:10.1073/pnas.0407686101
Chang DY, Lu AL (2005) Interaction of checkpoint proteins Hus1/Rad1/Rad9 with DNA base excision repair enzyme MutY homolog in fission yeast, Schizosaccharomyces pombe. J Biol Chem 280(1):408–417. doi:10.1074/jbc.M406800200
Friedrich-Heineken E, Toueille M, Tannler B, Burki C, Ferrari E, Hottiger MO, Hubscher U (2005) The two DNA clamps Rad9/Rad1/Hus1 complex and proliferating cell nuclear antigen differentially regulate flap endonuclease 1 activity. J Mol Biol 353(5):980–989. doi:10.1016/j.jmb.2005.09.018
Smirnova E, Toueille M, Markkanen E, Hubscher U (2005) The human checkpoint sensor and alternative DNA clamp Rad9-Rad1-Hus1 modulates the activity of DNA ligase I, a component of the long-patch base excision repair machinery. Biochem J 389(Pt 1):13–17. doi:10.1042/BJ20050211
Wang W, Lindsey-Boltz LA, Sancar A, Bambara RA (2006) Mechanism of stimulation of human DNA ligase I by the Rad9-rad1-Hus1 checkpoint complex. J Biol Chem 281(30):20865–20872. doi:10.1074/jbc.M602289200
Guan X, Bai H, Shi G, Theriot CA, Hazra TK, Mitra S, Lu AL (2007) The human checkpoint sensor Rad9-Rad1-Hus1 interacts with and stimulates NEIL1 glycosylase. Nucleic Acids Res 35(8):2463–2472. doi:10.1093/nar/gkm075
Gembka A, Toueille M, Smirnova E, Poltz R, Ferrari E, Villani G, Hubscher U (2007) The checkpoint clamp, Rad9-Rad1-Hus1 complex, preferentially stimulates the activity of apurinic/apyrimidinic endonuclease 1 and DNA polymerase beta in long patch base excision repair. Nucleic Acids Res 35(8):2596–2608. doi:10.1093/nar/gkl1139
Guan X, Madabushi A, Chang DY, Fitzgerald ME, Shi G, Drohat AC, Lu AL (2007) The human checkpoint sensor Rad9-Rad1-Hus1 interacts with and stimulates DNA repair enzyme TDG glycosylase. Nucleic Acids Res 35(18):6207–6218. doi:10.1093/nar/gkm678
Dore AS, Kilkenny ML, Rzechorzek NJ, Pearl LH (2009) Crystal structure of the rad9-rad1-hus1 DNA damage checkpoint complex–implications for clamp loading and regulation. Mol Cell 34(6):735–745. doi:10.1016/j.molcel.2009.04.027
Wu X, Shell SM, Yang Z, Zou Y (2006) Phosphorylation of nucleotide excision repair factor xeroderma pigmentosum group A by ataxia telangiectasia mutated and Rad3-related-dependent checkpoint pathway promotes cell survival in response to UV irradiation. Cancer Res 66(6):2997–3005. doi:10.1158/0008-5472.CAN-05-3403
Wu X, Shell SM, Liu Y, Zou Y (2007) ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation. Oncogene 26(5):757–764. doi:10.1038/sj.onc.1209828
Auclair Y, Rouget R, el Affar B, Drobetsky EA (2008) ATR kinase is required for global genomic nucleotide excision repair exclusively during S phase in human cells. Proc Natl Acad Sci USA 105(46):17896–17901. doi:10.1073/pnas.0801585105
Lee TH, Park JM, Leem SH, Kang TH (2012) Coordinated regulation of XPA stability by ATR and HERC2 during nucleotide excision repair. Oncogene 33(1):19–25. doi:10.1038/onc.2012.539
Li Z, Musich PR, Zou Y (2011) Differential DNA damage responses in p53 proficient and deficient cells: cisplatin-induced nuclear import of XPA is independent of ATR checkpoint in p53-deficient lung cancer cells. Int J Biochem Mol Biol 2(2):138–145
Auclair Y, Rouget R, Drobetsky EA (2009) ATR kinase as master regulator of nucleotide excision repair during S phase of the cell cycle. Cell Cycle 8(12):1865–1871
Patil M, Pabla N, Dong Z (2013) Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol Life Sci 70(21):4009–4021. doi:10.1007/s00018-013-1307-3
Yang XH, Shiotani B, Classon M, Zou L (2008) Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev 22(9):1147–1152. doi:10.1101/gad.1632808
Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T (2005) The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7(2):195–201. doi:10.1038/ncb1212
Brown KD, Rathi A, Kamath R, Beardsley DI, Zhan Q, Mannino JL, Baskaran R (2003) The mismatch repair system is required for S-phase checkpoint activation. Nat Genet 33(1):80–84. doi:10.1038/ng1052
Yoshioka K, Yoshioka Y, Hsieh P (2006) ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol Cell 22(4):501–510. doi:10.1016/j.molcel.2006.04.023
Arcangioli B, Ben Hassine S (2009) Unrepaired oxidative DNA damage induces an ATR/ATM apoptotic-like response in quiescent fission yeast. Cell Cycle 8(15):2326–2331
Brem R, Fernet M, Chapot B, Hall J (2008) The methyl methanesulfonate induced S-phase delay in XRCC1-deficient cells requires ATM and ATR. DNA Repair (Amst) 7(6):849–857. doi:10.1016/j.dnarep.2008.02.002
Marini F, Nardo T, Giannattasio M, Minuzzo M, Stefanini M, Plevani P, Muzi Falconi M (2006) DNA nucleotide excision repair-dependent signaling to checkpoint activation. Proc Natl Acad Sci USA 103(46):17325–17330. doi:10.1073/pnas.0605446103
Oh KS, Bustin M, Mazur SJ, Appella E, Kraemer KH (2011) UV-induced histone H2AX phosphorylation and DNA damage related proteins accumulate and persist in nucleotide excision repair-deficient XP-B cells. DNA Repair (Amst) 10(1):5–15. doi:10.1016/j.dnarep.2010.09.004
Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE (2006) H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci USA 103(26):9891–9896. doi:10.1073/pnas.0603779103
Ray A, Milum K, Battu A, Wani G, Wani AA (2013) NER initiation factors, DDB2 and XPC, regulate UV radiation response by recruiting ATR and ATM kinases to DNA damage sites. DNA Repair (Amst) 12(4):273–283. doi:10.1016/j.dnarep.2013.01.003
Colton SL, Xu XS, Wang YA, Wang G (2006) The involvement of ataxia-telangiectasia mutated protein activation in nucleotide excision repair-facilitated cell survival with cisplatin treatment. J Biol Chem 281(37):27117–27125. doi:10.1074/jbc.M602826200
Sertic S, Pizzi S, Cloney R, Lehmann AR, Marini F, Plevani P, Muzi-Falconi M (2011) Human exonuclease 1 connects nucleotide excision repair (NER) processing with checkpoint activation in response to UV irradiation. Proc Natl Acad Sci USA 108(33):13647–13652. doi:10.1073/pnas.1108547108
Lindsey-Boltz LA, Kemp MG, Reardon JT, Derocco V, Iyer RR, Modrich P, Sancar A (2014) Coupling of human DNA excision repair and the DNA damage checkpoint in a defined in vitro system. J Biol Chem 289(8):5074–5082. doi:10.1074/jbc.M113.542787
Stojic L, Brun R, Jiricny J (2004) Mismatch repair and DNA damage signalling. DNA Repair (Amst) 3(8–9):1091–1101. doi:10.1016/j.dnarep.2004.06.006
Franchitto A, Pichierri P, Piergentili R, Crescenzi M, Bignami M, Palitti F (2003) The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 22(14):2110–2120. doi:10.1038/sj.onc.1206254
Liu A, Yoshioka K, Salerno V, Hsieh P (2008) The mismatch repair-mediated cell cycle checkpoint response to fluorodeoxyuridine. J Cell Biochem 105(1):245–254. doi:10.1002/jcb.21824
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3(5):330–338. doi:10.1038/nrc1074
An Q, Robins P, Lindahl T, Barnes DE (2007) 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Res 67(3):940–945. doi:10.1158/0008-5472.CAN-06-2960
Wang Y, Qin J (2003) MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc Natl Acad Sci USA 100(26):15387–15392. doi:10.1073/pnas.2536810100
Stojic L, Mojas N, Cejka P, Di Pietro M, Ferrari S, Marra G, Jiricny J (2004) Mismatch repair-dependent G2 checkpoint induced by low doses of SN1 type methylating agents requires the ATR kinase. Genes Dev 18(11):1331–1344. doi:10.1101/gad.294404
Liu Y, Fang Y, Shao H, Lindsey-Boltz L, Sancar A, Modrich P (2010) Interactions of human mismatch repair proteins MutSalpha and MutLalpha with proteins of the ATR-Chk1 pathway. J Biol Chem 285(8):5974–5982. doi:10.1074/jbc.M109.076109
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4(4):307–320. doi:10.1038/nrd1691
Pabla N, Huang S, Mi QS, Daniel R, Dong Z (2008) ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283(10):6572–6583. doi:10.1074/jbc.M707568200
Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z (2011) hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem 286(12):10411–10418. doi:10.1074/jbc.M110.210989
Williams RS, Williams JS, Tainer JA (2007) Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem Cell Biol 85(4):509–520. doi:10.1139/O07-069
Soutoglou E, Misteli T (2008) Activation of the cellular DNA damage response in the absence of DNA lesions. Science 320(5882):1507–1510. doi:10.1126/science.1159051
Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP (2007) Human CtIP promotes DNA end resection. Nature 450(7169):509–514. doi:10.1038/nature06337
Nakada D, Hirano Y, Sugimoto K (2004) Requirement of the Mre11 complex and exonuclease 1 for activation of the Mec1 signaling pathway. Mol Cell Biol 24(22):10016–10025. doi:10.1128/MCB.24.22.10016-10025.2004
Kastan MS, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432:316–323
Acknowledgments
The research in the Yan lab is supported in part by funds provided by University of North Carolina at Charlotte and a Grant from the NIGMS/NIH (R15 GM101571). We apologize to our colleagues whose publications were not cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, S., Sorrell, M. & Berman, Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell. Mol. Life Sci. 71, 3951–3967 (2014). https://doi.org/10.1007/s00018-014-1666-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1666-4