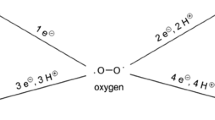
Abstract
The so-called reactive oxygen species (ROS) are defined as oxygen-containing species that are more reactive than O2 itself, which include hydrogen peroxide and superoxide. Although these are quite stable, they may be converted in the presence of transition metal ions, such as Fe(II), to the highly reactive oxygen species (hROS). hROS may exist as free hydroxyl radicals (HO·), as bound (“crypto”) radicals or as Fe(IV)-oxo (ferryl) species and the somewhat less reactive, non-radical species, singlet oxygen. This review outlines the processes by which hROS may be formed, their damaging potential, and the evidence that they might have signaling functions. Since our understanding of the formation and actions of hROS depends on reliable procedures for their detection, particular attention is given to procedures for hROS detection and quantitation and their applicability to in vivo studies.






Similar content being viewed by others
References
Jameson GNL, Jameson RF, Linert W (2004) New insights into iron release from ferritin: direct observation of the neurotoxin 6-hydroxydopamine entering ferritin and reaching redox equilibrium with the iron core. Org Biomol Chem 2:2346–2351
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Moskovitz J, Yim MB, Chock PB (2002) Free radicals and disease. Arch Biochem Biophys 397:354–359
Rush JD, Koppenol WH (1988) Reactions of iron(II) nitrilotriacetate and iron(II) ethylenediamine-N, N′-diacetate complexes with hydrogen peroxide. J Am Chem Soc 110:4957–4963
Walling C (1975) Fenton’s reagent revisited. Acc Chem Res 8:125–131
Lloyd RV, Hanna PM, Mason RP (1997) The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic Biol Med 22:885–888
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424
Szabó C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 8:662–680
Wood PM (1988) The potential diagram for oxygen at pH 7. Biochem J 253:287–289
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Ann Rev Biochem 77:755–776
Pace GW, Leaf CD (1995) The role of oxidative stress in HIV disease. Free Radic Biol Med 19:523–528
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R (2007) Diabetes-associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol 583:9–24
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Chinta SJ, Andersen JK (2008) Redox imbalance in Parkinson’s disease. Biochim Biophys Acta 1780:1362–1367
Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJ, Perstin J, Preston TJ, Wiley MJ, Wong AW (2009) Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci 108:4–18
Beckman KB, Ames BN (1988) The free radical theory of aging matures. Physiol Rev 78:547–581
Harman D (2006) Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci 1067:10–21
De la Fuente M (2002) Effects of antioxidants on immune system ageing. Eur J Clin Nutr 56(Suppl 3):S5–S8
Stone JR, Yang S (2004) Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8:243–270
D’Autréaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8:722–778
Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG (2010) Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140:517–528
Saran M, Michel C, Stettmaier K, Bors W (2000) Arguments against the significance of the Fenton reaction contributing to signal pathways under in vivo conditions. Free Radic Res 33:567–579
De Grey AD (2002) HO2*: the forgotten radical. DNA Cell Biol 21:251–257
Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW (1999) In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson’s and Alzheimer’s diseases. Biochim Biophys Acta 1454:143–152
Freinbichler W, Tipton KF, Della Corte L, Linert W (2009) Mechanistic aspects of the Fenton reaction under conditions approximated to the extracellular fluid. J Inorg Biochem 103:28–34
Wink DA, Wink CB, Nims RW, Ford PC (1994) Oxidizing intermediates generated in the Fenton reagent: kinetic arguments against the intermediacy of the hydroxyl radical. Environ Health Perspect 102(Suppl 3):11–15
Koppenol WH, Liebman JF (1984) The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2 +). J Phys Chem 88:99–101
Merkofer M, Kissner R, Hider RC, Brunk UT, Koppenol WH (2006) Fenton chemistry and iron chelation under physiologically relevant conditions: electrochemistry and kinetics. Chem Res Toxicol 19:1263–1269
Kremer ML (2000) Is *OH the active Fenton intermediate in the oxidation of ethanol? J Inorg Biochem 78:255–257
Halliwell B, Gutteridge JM (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett 307:108–112
Jameson GNL, Linert W (2001) The oxidation of 6-hydroxydopamine in aqueous solution. Part 2. Speciation and product distribution with iron(III) as oxidant. J Chem Soc, Perkin Trans 2:563–568
Jameson GNL, Kudryavtsev AB, Linert W (2001) The oxidation of 6-hydroxydopamine in aqueous solution. Part 1. The formation of three metastable quinones at low pH. J Chem Soc, Perkin Trans 2:557–562
Welch KD, Davis TZ, Aust StD (2002) Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch Biochem Biophys 397:360–369
Duesterberg CK, Cooper WJ, Waite T (2005) Fenton-mediated oxidation in the presence and absence of oxygen. Environ Sci Technol 39:5052–5058
Takeshita K, Ozawa T (2004) Recent progress in in vivo ESR spectroscopy. J Radiat Res 45:373–384
Kopáni M, Celec P, Danisovic L, Michalka P, Biró C (2006) Oxidative stress and electron spin resonance. Clin Chim Acta 364:61–66
Rush JD, Koppenol WH (1986) Oxidizing Intermediates in the reaction of ferrous EDTA with hydrogen peroxide. J Biol Chem 261:6730–6733
Di Matteo V, Pieruccia M, Di Giovanni G, Di Santo A, Poggia A, Benigno A, Esposito E (2006) Aspirin protects striatal dopaminergic neurons from neurotoxin-induced degeneration: an in vivo microdialysis study. Brain Res 1095:167–177
Yamazaki I, Piette LH (1990) ESR spin-trapping studies on the reaction of Fe2+ ions with H202-reactive species in oxygen toxicity in biology. J Biol Chem 265:13589–13594
Freinbichler W, Colivicchi MA, Fattori M, Ballini C, Tipton KF, Linert W, Della Corte L (2008) Validation of a robust and sensitive method for detecting hydroxyl radical formation together with evoked neurotransmitter release in brain microdialysis. J Neurochem 105:738–749
Freinbichler W, Bianchi L, Colivicchi MA, Ballini C, Tipton KF, Linert W, Della Corte L (2008) The detection of hydroxyl radicals in vivo. J Inorg Biochem 102:1329–1333
Schafer FQ, Qian SY, Buettner GR (2000) Iron and free radical oxidations in cell membranes. Cell Mol Biol (Noisy-le-grand) 46:657–662
Agil A, Fuller CJ, Jialal I (1995) Susceptibility of plasma to ferrous iron/hydrogen peroxide-mediated oxidation: demonstration of a possible Fenton reaction. Clin Chem 41:220–225
Witte I, Zhu BZ, Lueken A, Magnani D, Stossberg H, Chevion M (2000) Protection by desferrioxamine and other hydroxamic acids against tetrachlorohydroquinone-induced cyto- and genotoxicity in human fibroblasts. Free Radic Biol Med 28:693–700
Antunes F, Cadenas E (2001) Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med 30:1008–1018
Dikalova AE, Kadiiska MB, Mason RP (2001) An in vivo ESR spin-trapping study: free radical generation in rats from formate intoxication–role of the Fenton reaction. Proc Natl Acad Sci USA 98:13549–13553
Kuppusamy P, Zweier JL (1989) Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem 264:9880–9884
Britigan BE, Pou S, Rosen GM, Lilleg DM, Buettner GR (1990) Hydroxyl radical is not a product of the reaction of xanthine oxidase and xanthine. The confounding problem of adventitious iron bound to xanthine oxidase. J Biol Chem 265:17533–17538
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Szasz T, Thakali K, Fink GD, Watts SW (2007) A comparison of arteries and veins in oxidative stress: producers, destroyers, function, and disease. Exp Biol Med (Maywood) 232:27–37
Cohen MS, Britigan BE, Pou S, Rosen GM (1991) Application of spin trapping to human phagocytic cells: insight into conditions for formation and limitation of hydroxyl radical. Free Radic Res Commun 12:17–25
Hogg N, Darley-Usmar VM, Wilson MT, Moncada S (1992) Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J 281:419–424
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc NatI Acad Sci USA 87:1620–1624
Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE (1995) Free radicals and phagocytic cells. FASEB J 9:200–209
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625
Long CA, Bielski BH (1980) Rate of reaction of superoxide radical with chloride-containing species. J Phys Chem 84:555–557
Ramos CL, Pou S, Britigan BE, Cohen MS, Rosen GM (1992) Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem 267:8307–8312
Candeias LP, Patel KB, Stratford MR, Wardman P (1993) Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett 333:151–153
Chen SX, Schopfer P (1999) Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem 260:726–735
Kellogg EW, Fridovich I (1975) Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem 250:8812–8817
Khan AU, Kasha M (1994) Singlet molecular oxygen in the Haber-Weiss reaction. Proc Natl Acad Sci USA 91:12365–12367
Martinez GR, Di Mascio P, Bonini MG, Augusto O, Briviba K, Sies H, Maurer P, Röthlisberger U, Herold S, Koppenol WH (2000) Peroxynitrite does not decompose to singlet oxygen (1DgO2) and nitroxyl (NO−). Proc Natl Acad Sci USA 97:10307–10312
Kumar V, Tripathi MR, Kumar M, Shukla G, Dwivedi S, Sharma V (2009) Studies on production and chemical property of singlet oxygen and superoxide radical by dyestuffs. E-J Chem 6(S1):S79–S86
Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR (2006) Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, singlet oxygen sensor green. J Exp Bot 57:1725–1734
Xia Q, Yin JJ, Fu PP, Boudreau MD (2007) Photo-irradiation of Aloe vera by UVA-formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. Toxicol Lett 168:165–175
Fu PP, Xia Q, Yin JJ, Cherng SH, Yan J, Mei N, Chen T, Boudreau MD, Howard PC, Wamer WG (2007) Photodecomposition of vitamin A and photobiological implications for the skin. Photochem Photobiol 83:409–424
Egorov SY, Krasnovsky AA Jr, Bashtanov MY, Mironov EA, Ludnikova TA, Kritsky MS (1999) Photosensitization of singlet oxygen formation by pterins and flavins. Time-resolved studies of oxygen phosphorescence under laser excitation. Biochemistry (Mosc) 64:1117–1121
Kanofsky JR, Sima P (1991) Singlet oxygen production from the reactions of ozone with biological molecules. J Biol Chem 266:9039–9042
Simon F, Varela D, Eguiguren AL, Díaz LF, Sala F, Stutzin A (2004) Hydroxyl radical activation of a Ca2+-sensitive non selective cation channel involved in epithelial cell necrosis. Am J Physiol Cell Physiol 287:963–970
Burlando B, Viarengo A (2005) Ca2+ is mobilized by hydroxyl radical but not by superoxide in RTH-149 cells: the oxidative switching-on of Ca2+ signalling. Cell Calcium 38:507–513
Murphy RM, Dutka TL, Lamb GD (2008) Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol 586:2203–2216
Ishii M, Shimizu S, Hara Y, Hagiwara T, Miyazaki A, Mori Y, Kiuchi Y (2006) Intracellular-produced hydroxyl radical mediates H2O2-induced Ca2+ influx and cell death in rat b-cell line RIN-5F. Cell Calcium 39:487–494
Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci 7:444–458
Forman HJ (2007) Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med 42:926–932
Keyer K, Imlay JA (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA 93:13635–13640
McCormick ML, Buettner GR, Britigan BE (1998) Endogenous superoxide dismutase levels regulate iron-dependent hydroxyl radical formation in Escherichia coli exposed to hydrogen peroxide. J Bacteriol 180:622–625
Liochev SI, Fridovich I (1994) The role of O2·− in the production of: in vitro and in vivo. Free Radic Biol Med 16:29–33
Davies MJ (2005) The oxidative environment and protein damage. Biochim Biophys Acta 1703:93–109
Rouault TA, Tong WH (2008) Iron–sulfur cluster biogenesis and human disease. Trends Genet 24:398–407
Schneider C, Porter NA, Brash AR (2008) Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem 283:15539–15543
Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC (2007) Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep 12:4–10
Catalá A (2009) Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 157:1–11
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57(5 Suppl):715S–724S
Rahman I, Biswas SK (2004) Non-invasive biomarkers of oxidative stress: reproducibility and methodological issues. Redox Rep 9:125–143
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316
Davies MJ (2005) The oxidative environment and protein damage. Biochim Biophys Acta 1703:93–109
Gracanin M, Hawkins CL, Pattison DI, Davies MJ (2009) Singlet-oxygen-mediated amino acid and protein oxidation: formation of tryptophan peroxides and decomposition products. Free Radic Biol Med 47:92–102
Zhang X-H (2010) Regulation of protein function by residue oxidation. Proteomics Insights 3:17–24
Bergeron F, Auvré F, Radicella JP, Ravanat JL (2010) HO* radicals induce an unexpected high proportion of tandem base lesions refractory to repair by DNA glycosylases. Proc Natl Acad Sci USA 107:5528–5533
Cavalcante AK, Martinez GR, Di Mascio P, Menck CF, Agnez-Lima LF (2002) Cytotoxicity and mutagenesis induced by singlet oxygen in wild type and DNA repair deficient Escherichia coli strains. DNA Repair (Amst) 1:1051–1056
Mishra PC, Singh AK, Suha S (2005) Interaction of singlet oxygen and superoxide radical anion with guanine and formation of its mutagenic modification 8-oxoguanine. Int J Quantum Chem 102:282–301
Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M (2007) Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104:8749–8754
Jensen SJ, Csizmadia IG (2001) Hydroxyl radicals piggybacking on hydrogen carbonate. Phys Chem Lett 431:633–637
Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z (2003) Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol 50:1129–1146
Buettner GR, Jurkiewicz BA (1996) Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res 145:532–541
Mishin VM, Thomas PE (2004) Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem Pharmacol 68:747–752
Yan EB, Unthank JK, Castillo-Melendez M, Miller SL, Langford SJ, Walker DW (2005) A novel Method for in vivo hydroxyl radical measurement by microdialysis in fetal sheep brain, in utero. J Appl Physiol 98:2304–2310
Lang K, Wagnerova DM, Brodilova J (1994) The role of hydrogen peroxide in dioxygen induced hydroxylation of salicylic acid. Collect Czechoslov Chem Commun 59:2447–2453
Lunak S, Muzart J, Brodilova J (1994) Photochemical hydroxylation of salicylic acid derivatives with hydrogen peroxide, catalyzed with Fe(III) and sensitised with methylene blue. Collect Czechoslov Chem Commun 59:905–912
Maskos Z, Rush JD, Koppenol WH (1990) The hydroxylation of the salicylate anion by a Fenton reaction and T-radiolysis: a consideration of the respective mechanisms. Free Radic Biol Med 8:153–162
Salzberg-Brenhouse HC, Chen EY, Emerich DF, Baldwin S, Hogeland K, Ranelli S, Lafreniere D, Perdomo B, Novak L, Kladis T, Fu K, Basile AS, Kordower JH, Bartus RT (2003) Inhibitors of cyclooxygenase-2, but not cyclooxygenase-1 provide structural and functional protection against quinolinic acid-induced neurodegeneration. J Pharmacol Exp Ther 306:218–228
Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling TE, Langenbach R, Taniura S, Hong JS (2004) Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem 88:939–947
Catania A, Arnold J, Macaluso A, Hiltz ME, Lipton JM (1991) Inhibition of acute inflammation in the periphery by central action of salicylates. Proc Natl Acad Sci USA 88:8544–8547
Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM (2006) Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J 20:2009–2016
Themann C, Teismann P, Kuschinsky K, Ferger B (2001) Comparison of two independent aromatic hydroxylation assays in combination with intracerebral microdialysis to determine hydroxyl free radicals. J Neurosci Methods 108:57–64
Kaur H, Halliwell B (1994) Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals. Anal Biochem 220:11–15
Moreno S, Nardacci R, Cimini A, Ceru MP (1999) Immunocytochemical localization of d-amino acid oxidase in rat brain. J Neurocytol 28:169–185
Reddy S, Halliwel B, Jones AD, Longhurst JC (1999) The use of phenylalanine to detect hydroxyl radical production in vivo: a cautionary note. Free Radic Biol Med 27:1465
Ferger B, Themann C, Rose S, Halliwell B, Jenner P (2001) 6-Hydroxydopamine increases the hydroxylation and nitration of phenylalanine in vivo: implication of peroxynitrite formation. J Neurochem 78:509–514
Liu M, Liu S, Peterson SL, Miyake M, Liu KJ (2002) On the application of 4-hydroxybenzoic acid as a trapping agent to study hydroxyl radical generation during cerebral ischemia and reperfusion source. Mol Cell Biochem 234:379–385
Marklund N, Clausen F, Lewander T, Hillered L (2001) Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J Neurotrauma 18:1217–1227
Kalén A, Appelkvist E-L, Chojnacki T, Dallner G (1990) Nonaprenyl-4-hydroxybenzoate transferase, an enzyme involved in ubiquinone biosynthesis, in the endoplasmic reticulum–Golgi system of rat liver. J Biol Chem 265:1158–1164
Matthews RW (1980) The radiation chemistry of the terephthalate dosimeter. Radiat Res 83:27–41
Schimmel M, Bauer G (2002) Proapoptotic and redox state-related signaling of reactive oxygen species generated by transformed fibroblasts. Oncogene 21:5886–5896
Barreto JC, Smith GS, Strobel NH, McQuillin PA, Miller TA (1995) Terephthalic acid: a dosimeter for the detection of hydroxyl radicals in vitro. Life Sci 56:PL89–PL96
Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeira A, Muñoz-Patiño AM, Labandeira-Garcia JL (2000) Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson’s disease. J Neurochem 74:1605–1612
Saran M, Summer KH (1999) Assaying for hydroxyl radicals: hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free Radic Res 31:429–436
Dai GD, Cui LB, Song L, Zhao RZ, Chen JF, Wang YB, Chang HC, Wang XR (2006) Metabolism of terephthalic acid and its effects on CYP4B1 induction. Biomed Environ Sci 19:8–14
OECD SIDS (2001) Terephthalic acid (TPA): initial assessment report. For 12th SIAM (Paris, France June 2001), United Nations Environment Programme (UNEP) Publications, Nairobi
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
Togashi H, Shinzawa H, Matsuo T, Takeda Y, Takahashi T, Aoyama M, Oikawa K, Kamada H (2000) Analysis of hepatitic oxidative stress status by electron spin resonance spectroscopy and imaging. Free Radic Biol Med 28:846–853
Kopáni M, Celec P, Danisovic L, Michalka P, Biró C (2006) Oxidative stress and electron spin resonance. Clin Chim Acta 364:61–66
Swartz HM, Khan N, Khramtsov VV (2007) Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxid Redox Signal 9:1757–1771
Ueda Y, Doi T, Nagatomo K, Nakajim A (2007) In vivo activation of N-methyl-d-aspartate receptors generates free radicals and reduces antioxidant ability in the rat hippocampus: experimental protocol of in vivo ESR spectroscopy and microdialysis for redox status evaluation. Brain Res 1178:20–27
Gallez B, Swartz HM (2004) In vivo EPR: when, how and why? NMR Biomed 17:223–225
Utsumi H, Yasukawa K, Soeda T, Yamada KI, Shigemi R, Yao T, Tsuneyoshi M (2006) Noninvasive mapping of reactive oxygen species by in vivo electron spin resonance spectroscopy in indomethacin-induced gastric ulcers in rats. J Pharmacol Exp Ther 317:228–235
Halliwell B (1999) Antioxidant defence mechanisms: from the beginning to the end of the beginning. Free Radic Res 31:261–272
Kohen P, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650
Kim C, Lee KP, Baruah A, Nater M, Göbel C, Feussner I, Apel K (2009) 1O2-mediated retrograde signaling during late embryogenesis predetermines plastid differentiation in seedlings by recruiting abscisic acid. Proc Natl Acad Sci USA 106:9920–9924
Obata T, Inada T, Yamanaka Y (1997) Intracranial microdialysis of salicylic acid to detect hydroxyl radical generation by antidepressant drugs in the rat. Neurosci Res Commun 21:223–229
Camarero J, Sanchez V, O’Shea E, Green AR, Colada MI (2002) Studies, using in vivo microdialysis, on the effect of the dopamine uptake inhibitor GBR 12909 on 3, 4-methylenedioxymethamphetamine (‘ecstasy’)-induced dopamine release and free radical formation in the mouse striatum. J Neurochem 81:961–972
Mason RB, Pluta RM, Walbridge S, Wink DA, Oldfield EH, Boock RJ (2000) Production of reactive oxygen species after reperfusion in vitro and in vivo: protective effect of nitric oxide. J Neurosurg 93:99–107
Montgomery J, Ste-Marie L, Boismenu D, Vachon L (1995) Hydroxylation of aromatic compounds as indices of hydroxyl radical production: a cautionary note revisited. Free Radic Biol Med 19:927–933
Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL (1999) An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods 90:129–142
Acknowledgments
We are grateful for financial support from “Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich” (Project 19335-N17), Ente Cassa di Risparmio di Firenze (Firenze, Italy), Università degli Studi di Firenze, Science Foundation Ireland, ERAB: The European Foundation for Alcohol Research (Brussels, Belgium), and also to the EU COST action D34 supporting our international cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freinbichler, W., Colivicchi, M.A., Stefanini, C. et al. Highly reactive oxygen species: detection, formation, and possible functions. Cell. Mol. Life Sci. 68, 2067–2079 (2011). https://doi.org/10.1007/s00018-011-0682-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0682-x