Abstract
Objective and methods
Nitrogen-containing bisphosphonates (NBPs, anti-bone-resorptive agents) have inflammatory side-effects. Alendronate (Ale, an NBP) intradermally injected into mouse ear-pinnae together with LPS (bacterial cell-wall component) induces augmented ear-swelling that depends on IL-1 and neutrophils. Using this model, we examined histamine’s involvement in Ale + LPS-induced inflammation.
Results
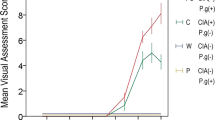
Ale increased histamine in ear-pinnae by inducing histidine decarboxylase (HDC). This induction was augmented by LPS. In HDC-deficient mice, such augmented ear-swelling was not induced. At peak-swelling, 74.5% of HDC-expressing cells were neutrophils and only 0.2% were mast cells (MCs). The augmented swelling was markedly reduced by a histamine H4-receptor (H4R) antagonist, but not by an H1R antagonist. In MC-deficient mice, unexpectedly, Ale + LPS induced prolonged ear-swelling that was augmented and more persistent than in normal mice. MCs highly expressed H4Rs and produced MCP-1(inflammatory cytokine that recruits macrophages) and IL-10 (anti-inflammatory cytokine) in response to an H4R agonist.
Conclusion
Histamine produced by HDC-induction mainly in infiltrated neutrophils stimulates H4Rs, leading to augmented Ale + LPS-induced ear-swelling via MCP-1 production by MCs. Since MCP-1 is produced by other cells, too, the contribution of MCs and their H4Rs to augmented ear-swelling is partial. In the later phase of the swelling, MCs may be anti-inflammatory via IL-10 production.







Similar content being viewed by others
References
Fleisch H, Russell RGG, Bisaz S, Casey PA, Mühlbauer RC. The influence of pyrophosphate analogues (diphosphonates) on the precipitation and dissolution of calcium phosphate in vitro and in vivo. Calcif Tissue Res. 1968;2(S1):10–10a.
Russell RGG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19.
Coleman R. Bisphosphonates and breast cancer - from cautious palliation to saving lives. Bone. 2020;140: 115570.
Billington EO, Reid IR. Benefits of bisphosphonate therapy: beyond the skeleton. Curr Osteoporos Rep. 2020;18:587–96.
Rogers MJ, Mönkkönen J, Munoz MA. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone. 2020;139: 115493.
Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo CV. The acute phase response after bisphosphonate administration. Calcif Tissue Int. 1987;41:326–31.
Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7.
Green J, Czanner G, Reeves G, Watoson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341: c4444.
Lengfeld J, Buder-Bakhaya K, Goebeler M, Wobser M. Bisphosphonate-mediated oral ulcers: a rare differential diagnosis of erosive oral lesions. Dermatology. 2016;232:117–21.
McCadden L, Leonard CG, Primrose WJ. Bisphosphonate-induced osteonecrosis of the ear canal: our experience and a review of the literature. J Laryngol Otol. 2018;132:372–4.
Endo Y, Nakamura M, Kikuchi T, Shinoda H, Takeda Y, Nitta Y, Kumagai K. Aminoalkyl bisphosphonates, potent inhibitors of bone resorption, induce a prolonged stimulation of histamine synthesis and increase macrophages, granulocytes and osteoclasts in vivo. Calcif Tissue Int. 1993;52:248–54.
Sugawara S, Shibazaki M, Takada H, Kosugi H, Endo Y. Contrasting effects of an aminobisphosphonate, a potent inhibitor of bone resorption, on lipopolysaccharide-induced production of interleukin-1 and tumor necrosis factor α in mice. Br J Pharmacol. 1998;125:735–40.
Deng X, Yu Z, Funayama H, Shoji N, Sasano T, Iwakura Y, Sugawara S, Endo Y. Mutual augmentation of the induction of the histamine-forming enzyme, histidine decarboxylase, between alendronate and immuno-stimulants (IL-1, TNF, and LPS), and its prevention by clodronate. Toxicol Appl Pharmacol. 2006;213:64–73.
Shikama Y, Nagai Y, Okada S, Oizumi T, Shimauchi H, Sugawara S, Endo Y. Pro-IL-1β accumulation in macrophages by alendronate and its prevention by clodronate. Toxicol Lett. 2010;199:123–8.
Okada S, Kiyama T, Sato E, Tanaka Y, Oizumi T, Kuroishi T, Takahashi T, Sasaki K, Sugawara S, Endo Y. Inhibition of phosphate transporters ameliorate the inflammatory and necrotic side effects of the nitrogen-containing bisphosphonate zoledronate in mice. Tohoku J Exp Med. 2013;231:145–58.
Funayama H, Tashima I, Okada S, Ogawa T, Yagi H, Tada H, Wakita R, Asada Y, Endo Y. Effects of zoledronate on local and systemic production of IL-1β, IL-18, and TNF-α in mice and augmentation by lipopolysaccharide. Biol Pharm Bull. 2019;42:929–36.
Bando K, Kuroishi T, Tada H, Oizumi T, Tanaka Y, Takahashi T, Mizoguchi I, Sugawara S, Endo Y. Nitrogen-containing bisphosphonates and lipopolysaccharide mutually augment inflammation via adenosine triphosphate (ATP)-mediated and interleukin 1β (IL-1β)-mediated production of neutrophil extracellular traps (NETs). J Bone Miner Res. 2021;36:1866–78.
Norton JT, Hayashi T, Crain B, Corr M, Carson DA. Role of IL-1 receptor-associated kinase-M (IRAK-M) in priming of immune and inflammatory responses by nitrogen bisphosphonates. Proc Natl Acad Sci USA. 2011;108:11163–8.
Norton JT, Hayashi T, Crain B, Cho JS, Miller LS, Corr M, Carson DA. Nitrogen bisphosphonate-induced inflammation is dependent upon mast cells and IL-1. J Immunol. 2012;188:2977–80.
Sakaguchi O, Kokuryo S, Tsurushima H, Tanaka J, Habu M, Uehara M, Nishihara T, Tominaga K. Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis in rats. Int J Oral Maxillofac Surg. 2015;44:528–34.
Hellstein JW, Marek CL. Bisphosphonate osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg. 2005;63:682–9.
Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508–14.
Endo Y, Kumamoto H, Nakamura M, Sugawara S, Takano-Yamamoto T, Sasaki K, Takahashi T. Underlying mechanisms and therapeutic strategies for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Biol Pharm Bull. 2017;40:739–50.
Sampson HA, Jolie PL. Increased plasma histamine concentrations after food challenges in children with atopic dermatitis. N Engl J Med. 1984;311:372–6.
Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, Park H, Shykind B, Diacovo TG, Falus A, Wang TC. Histamine deficiency promotes inflammation associated carcinogenesis through reduced myeloid maturation and accumulation of xCD11b+Ly6G+ immature myeloid cells. Nat Med. 2011;17:87–95.
Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. 2018;13:1873.
Bando K, Kuroishi T, Sugawara S, Endo Y. Interleukin-1 and histamine are essential for inducing nickel allergy in mice. Clin Exp Allergy. 2019;49:1362–73.
Walter M, Stark H. Histamine receptor subtypes: a century of rational drug design. Front in Biosci. 2012;4:461–88.
Tiligata E, Ennis M. Histamine pharmacology: from Sir Henry Dale to the 21st century. Br J Pharmacol. 2020;177:469–89.
Endo Y. Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochem Pharmacol. 1982;31:1643–7.
Endo Y. Induction of histidine decarboxylase in mouse tissues by mitogens in vivo. Biochem Pharmacol. 1983;32:3835–8.
Endo Y, Takeda Y, Nitta Y, Rikiishi H, Kumagai K. GM-CSF and G-CSF stimulates the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol Lett. 1992;33:9–14.
Wu X, Yoshida A, Sasano T, Iwakura Y, Endo Y. Histamine production via mast cell-independent induction of histidine decarboxylase in response to lipopolysaccharide and interleukin-1. Int Immunopharmacol. 2004;4:513–20.
Deng X, Yu Z, Funayama H, Yamaguchi K, Sasano T, Sugawara S, Endo Y. Histidine decarboxylase-stimulating and inflammatory effects of alendronate in mice: involvement of mevalonate pathway, TNFα, macrophages, and T-cells. Int Immunopharmacol. 2007;7:152–61.
Yamaguchi K, Motegi K, Iwakura Y, Endo Y. Involvement of interleukin-1 in the inflammatory actions of aminobisphosphonates in mice. Br J Pharmacol. 2000;130:1646–54.
Otsuka H, Endo Y, Ohtsu H, Inoue S, Noguchi S, Nakamura M, Soeta S. Histidine decarboxylase deficiency inhibits NBP-induced extramedullary hematopoiesis by modifying bone marrow and spleen microenvironments. Int J Hematol. 2021;113:348–61.
Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzás E, Kovács P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–6.
Takai J, Ohtsu H, Sato A, Uemura S, Fujimura T, Yamamoto M, Moriguchi T. Lipopolysaccharide-induced expansion of histidine decarboxylase-expressing Ly6G+ myeloid cells identified by exploiting histidine decarboxylase BAC-GFP transgenic mice. Sci Rep. 2019;9:15603.
Endo Y. A simple method for the determination of polyamines and histamine and its application to the assay of ornithine decarboxylase and histidine decarboxylase activities. Methods Enzymol. 1983;94:42–7.
Abd-Allah ARA, Ahmad SF, Alrashidi I, Abdel-Hamied HE, Zoheir KMA, Ashour AE, Bakheet SA, Attia SM. Involvement of histamine 4 receptor in the pathogenesis and progression of rheumatoid arthritis. Int Immunol. 2014;26:325–40.
Ahmad SF, Ansari MA, Zoheir KMA, Bakheet SA, Korashy HM, Nadeem A, Ashour AE, Attia SM. Regulation of TNF-α and NF-κB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology. 2015;220:889–98.
Rossbach K, Wahle K, Bruer G, Brehm R, Langeheine M, Rode K, Schaper-Gerhardt K, Gutzmer R, Werfel T, Kietzmann M, Bäumer W. Histamine 2 receptor agonism and histamine 4 receptor antagonism ameliorate inflammation in a model of psoriasis. Acta Derm Venereol. 2020;100:adv00342.
Bando K, Tanaka Y, Kuroishi T, Sasaki K, Takano-Yamamoto T, Sugawara S, Endo Y. Mouse model of hydroquinone hypersensitivity via innate and acquired immunity, and its promotion by combined reagents. J Invest Dermatol. 2017;137:1082–93.
Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188:5428–37.
Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104.
Hirasawa N. Expression of histidine decarboxylase and its roles in inflammation. Int J Mol Sci. 2019;20:376.
Huang H, Li Y, Liang J, Finkelman FD. Molecular regulation of histamine synthesis. Front Immunol. 2018;20:1392.
Schirmer B, Neumann D. The function of the histamine H4 receptor in inflammatory and inflammation-associated diseases of the gut. Int J Mol Sci. 2021;22:6116.
Endo Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochem Pharmacol. 1989;38:1287–92.
Suzuki-Ishigaki S, Numayama-Tsuruta K, Kuramasu A, Sakurai E, Makabe Y, Shimura S, Shirato K, Igarashi K, Watanabe T, Ohtsu H. The mouse L-histidine decarboxylase gene: structure and transcriptional regulation by CpG methylation in the promoter region. Nucleic Acids Res. 2000;28:2627–33.
Hirasawa N, Torigoe M, Kano K, Ohuchi K. Involvement of Sp1 in lipopolysaccharide induced expression of HDC mRNA in RAW 264 cells. Biochem Biophys Res Commun. 2006;349:833–7.
Shiraishi M, Hirasawa N, Oikawa S, Kobayashi Y, Ohuchi K. Analysis of histamine-producing cells at the late phase of allergic inflammation in rats. Immunology. 2000;99:600–7.
Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, Lowell CA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203:2907–17.
Xu X, Zhang H, Song Y, Lynch SV, Lowell CA, Wiener-Kronish JP, Caughey GH. Strain-dependent induction of neutrophil histamine production and cell death by Pseudomonas aeruginosa. J Leukoc Biol. 2012;91:275–84.
Alcañiz L, Vega A, Chacón P, Bekay RE, Ventura I, Aroca R, Blanca M, Bergstralh DT, MonteseirinJ. Histamine production by human neutrophils. FASEB J. 2013;27:2902–10.
Endo Y, Nakamura M, Nitta Y, Kumagai K. Effects of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin-1 and tumor necrosis factor. Br J Pharmacol. 1995;114:187–93.
Mehta P, Miszta P, Rzodkiewicz P, Michalak O, Krzeczynski P, Filipek S. Enigmatic histamine receptor H4 for potential treatment of multiple inflammatory, autoimmune, and related diseases. Life. 2020;10:50.
Schaper-Gerhardt K, Rossbach K, Nikolouli E, Werfel T, Gutzmer R, Mommert S. The role of the histamine H(4) receptor in atopic dermatitis and psoriasis. Br J Pharmacol. 2020;177:490–502.
Liu C, Wilson SJ, Kuei C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001;299:121–30.
Nicoud MB, Sterle HA, Massari NA, Delgado MAT, Formoso K, Ducloux MVH, Lamas DM, Cremaschi GA, Medin VA. Study of the antitumour effects and the modulation of immune response by histamine in breast cancer. Br J Cancer. 2020;122:348–60.
Dib K, Perecko T, Jenei V, McFarlane C, Comer D, Brown V, Katebe M, Scheithauer T, Thurmond RL, Chazot PL, Ennis M. The histamine H4 receptor is a potent inhibitor of adhesion-dependent degranulation in human neutrophils. J Leukoc Biol. 2014;96:411–8.
Zhou E, Wu Z, Zhu X, Li P, Wang J, Yang Z. Histamine triggers the formation of neutrophil extracellular traps via NADPH oxidase, ERK and p38 pathways. Vet Immunol Immunopathol. 2021;235: 110234.
Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung W-P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–21.
Jemima EA, Prema A, Thangam EB. Functional characterization of histamine H4 receptor on human mast cells. Mol Immunol. 2014;62:19–28.
Desai P, Thurmond RL. Histamine H4 receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur J Immunol. 2011;41:1764–73.
Sato N, Kinbara M, Kuroish T, Kimura K, Iwakura Y, Ohtsu H, Sugawara S, Endo Y. Lipopolysaccharide promotes and augments metal allergies in mice, dependent on innate immunity and histidine decarboxylase. Clin Exp Allergy. 2007;37:743–51.
Oizumi T, Funayama H, Yamaguchi K, Yokoyama M, Takahashi H, Yamamoto M, Kuroishi T, Kumamoto H, Sasaki K, Kawamura H, Sugawara S, Endo Y. Inhibition of necrotic actions of nitrogen-containing bisphosphonates (NBPs) and their elimination from bone by etidronate (a Non-NBP): a proposal for possible utilization of etidronate as a substitution drug for NBPs. J Oral Maxillof Surg. 2010;68:1043–54.
Acknowledgements
The authors thank Dr. Robert Timms for his language-editing. We thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for the use of its equipment. We thank Dr. Takashi Moriguchi and Dr. Jun Takai for providing HDC-GFP mice.
Funding
Research funding provided by Japan Society for Promotion of Science (18K17240, 21K10157 to KB).
Author information
Authors and Affiliations
Contributions
Study designed and conducted by KB, YE, YT, TT, IM, and SS. Data collected by KB. Data analyzed by KB and YE. Technically assisted by YT, YE, and SS. Manuscript written by KB and YE and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Tohoku University (approval number: 2019DnA-044, 2020DnLMO-013–01).
Consent for publication
Not applicable.
Additional information
Responsible Editor: Bernhard Gibbs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bando, K., Tanaka, Y., Takahashi, T. et al. Histamine acts via H4-receptor stimulation to cause augmented inflammation when lipopolysaccharide is co-administered with a nitrogen-containing bisphosphonate. Inflamm. Res. 71, 1603–1617 (2022). https://doi.org/10.1007/s00011-022-01650-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01650-7