Abstract
Objective and design
The omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) has been reported to suppress inflammation. Pulmonary inflammation can be directly linked to exposure of various occupational and man-made particles leading to pulmonary diseases. Therapeutic treatments are lacking for particle-induced pulmonary inflammation. These studies evaluated DHA as a therapeutic treatment for semi-acute and chronic particle-induced pulmonary inflammation.
Methods
Balb/c mice were oropharyngeal instilled with hydrophobic multi-walled carbon nanotube (MWCNT) or hydrophilic crystalline silica (SiO2) either as one instillation (semi-acute) or once a week for 4 weeks (chronic). One week later, the mice were placed on either a control or 1% DHA-containing diet for 3 weeks (semi-acute) or 12 weeks (chronic). Mice were assessed for inflammatory signaling within the lung lavage fluid, impact on phagolysosomal membrane permeability, shifts of macrophage phenotype gene expression (M1, M2a, M2b, and M2c), and pulmonary histopathology.
Results
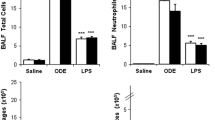
DHA increased pulmonary inflammatory markers and lung pathology when mice were exposed to SiO2. There were trending decreases of inflammatory markers for MWCNT-exposed mice with DHA treatment, however, mostly not statistically significant.
Conclusion
The anti-inflammatory benefits of DHA treatment depend upon the type of inflammatory particle, magnitude of inflammation, and duration of treatment.










Similar content being viewed by others
Availability of data and materials
Data and material will be provided by the authors if requested.
References
Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–15. https://doi.org/10.1042/BST20160474.
Molendi-Coste O, Legry V, Leclercq IA. Why and how meet n-3 PUFA dietary recommendations? Gastroenterol Res Pract. 2011. https://doi.org/10.1155/2011/364040.
Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3:1–7. https://doi.org/10.3945/an.111.000893.
Bates MA, Brandenberger C, Langohr II, Kumagai K, Lock AL, Harkema JR, Holian A, Pestka JJ. Silica-triggered autoimmunity in lupus-prone mice blocked by docosahexaenoic acid consumption. PLoS ONE. 2016;11(8):e0160622. https://doi.org/10.1371/journal.pone.0160622.
Li X-Y, Hao L, Liu Y-H, Chen C-Y, Pai VJ, Kang JX. Protection against fine particle-induced pulmonary and systemic inflammation by omega-3 polyunsaturated fatty acids. Biochim Biophys Acta. 2017;1861:577–84. https://doi.org/10.1016/j.bbagen.2016.12.018.
Forum of International Respiratory Societies. The global impact of respiratory disease. 2nd ed. Sheffield: European Respiratory Society; 2017.
Wong J, Magun BE, Wood LJ. Lung inflammation caused by inhaled toxicants: a review. Int J Chronic Obstr Pulm Dis. 2016;11:1391–401. https://doi.org/10.2147/COPD.S106009.
Lam C, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. https://doi.org/10.1080/10408440600570233.
Ray JL, Holian A. Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica. Inhal Toxicol. 2019;31(7):285–97. https://doi.org/10.1080/08958378.2019.1669743.
Pollard KM. Silica, silicosis, and autoimmunity. Front Immunol. 2016. https://doi.org/10.3389/fimmu.2016.00097.
Pavan C, Santalucia R, Leinardi R, Fabbiani M, Yakoub Y, Fubini B, et al. Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc Natl Acad Sci. 2020;117(45):27836–46. https://doi.org/10.1073/pnas.2008006117.
Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in TH1- and TH2-dominant mouse strains. Shock. 2004;22(5):460–6. https://doi.org/10.1097/01.shk.0000142249.08135.e9.
Limjunyawong N, Craig JM, Lagassé HAD, Scott AL, Mitzner W. Experimental progressive emphysema in BALB/cJ mice as a model for chronic alveolar destruction in humans. Am J Physiol Lung Cell Mol Physiol. 2015;309:L662–76. https://doi.org/10.1152/ajplung.00214.2015.
Sahu N, Morales JL, Fowell D, August A. Modeling susceptibility versus resistance in allergic airway disease reveals regulation by Tec kinase Itk. PLoS ONE. 2010;5(6):e11348. https://doi.org/10.1371/journal.pone.0011348.
Hamilton RF, Buford M, Xiang C, Wu N, Holian A. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal Toxicol. 2012;24(14):995–1008. https://doi.org/10.3109/08958378.2012.745633.
Thakur SA, Hamilton R, Pikkarainen T, Holian A. Differential binding of inorganic particles to MARCO. Toxicol Sci. 2009;107(1):238–46. https://doi.org/10.1093/toxsci/kfn210.
EFSA Nda Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the extension of use for DHA and EPA-rich algal oil from Schizochytrium sp as a novel food ingredient. Eur Food Saf Auth J. 2014;12(10):3843. https://doi.org/10.2903/j.efsa.2014.3843.
Jessop F, Hamilton RF, Rhoderick JF, Fletcher P, Holian A. Phagolysosome acidification is required for silica and engineered nanoparticle-induced lysosome membrane permeabilization and resultant NLRP3 inflammasome activity. Toxicol Appl Pharmacol. 2017;318:58–68. https://doi.org/10.1016/j.taap.2017.01.012.
Jessop F, Holian A. Extracellular HMGB1 regulates multi-walled carbon nanotube-induced inflammation in vivo. Nanotoxicology. 2015;9(3):365–72. https://doi.org/10.3109/17435390.2014.933904.
Burmeister R, Rhoderick JF, Holian A. Prevention of crystalline silica-induced inflammation by the anti-malarial hydroxychloroquine. Inhal Toxicol. 2019;31(7):274–84. https://doi.org/10.1080/08958378.2019.1668091.
Fletcher P, Hamilton RF, Rhoderick JF, Pestka JJ, Holian A. Docosahexaenoic acid impacts macrophage phenotype subsets and phagolysosomal membrane permeability with particle exposure. J Toxicol Environ Health Part A. 2020. https://doi.org/10.1080/15287394.2020.1842826.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. https://doi.org/10.1016/j.cell.2010.01.040.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. https://doi.org/10.1038/nature10759.
Labonte AC, Tosello-Trampont A-C, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells. 2014;37(4):275–85. https://doi.org/10.14348/molcells.2014.2374.
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014. https://doi.org/10.3389/fimmu.2014.00514.
Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax. 2015;70:1189–96. https://doi.org/10.1136/thoraxjnl-2015-207020.
Saifuddin N, Raziah AZ, Junizah AR. Carbon nanotubes: a review on structure and their interaction with proteins. J Chem. 2013. https://doi.org/10.1155/2013/676815.
Dyachenko AG, Borysenko MV, Pakhovchyshyn SV. Hydrophilic/hydrophobic properties of silica surfaces modified with metal oxides and polydimethylsiloxane. Adsorpt Sci Technol. 2004;22(6):511–6. https://doi.org/10.1260/0263617042879546.
Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):43–7. https://doi.org/10.1016/j.plefa.2012.03.008.
Wassall SR, Leng X, Canner SW, Pennington ER, Kinnun JJ, Cavazos AT, Dadoo S, Johnson D, Heberle FA, Katsaras J. Shaikh SR Docosahexaenoic acid regulates the formation of lipid rafts: a unified view from experiment and simulation. Biochim Biophys Acta Biomembr. 2018;1860(10):1985–93. https://doi.org/10.1016/j.bbamem.2018.04.016.
Stunault MI, Bories G, Guinamard RR, Ivanov S. Metabolism plays a key role during macrophage activation. Mediat Inflamm. 2018. https://doi.org/10.1155/2018/2426138.
Li R, Qiu X, Xu F, Lin Y, Fang Y, Zhu T. Macrophage-mediated effects of airborne fine particulate matter (PM2.5) on hepatocyte insulin resistance in vitro. ACS Omega. 2016;1:736–43. https://doi.org/10.1021/acsomega.6b00135.
Finucane OM, Sugrue J, Rubio-Araiz A, Guillot-Sestier M-V, Lynch MA. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci Rep. 2019;9:4034. https://doi.org/10.1038/s41598-019-40619-1.
Ali M, Heyob K, Rogers LK. DHA suppresses primary macrophage inflammatory responses via notch 1/ jagged 1 signaling. Sci Rep. 2016;6:22276. https://doi.org/10.1038/srep22276.
Benmoussa K, Garaude J, Acín-Pérez R. How mitochondrial metabolism contributes to macrophage phenotype and functions. J Mol Biol. 2018;430:3906–21. https://doi.org/10.1016/j.jmb.2018.07.003.
Shen L, Yang Y, Ou T, Key C-CC, Tong SH, Zhu X, et al. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J Lipid Res. 2017;58:1808–21. https://doi.org/10.1194/jlr.M075879.
Saitoh T, Fujita N, Jang MH, Uematsu S, Yang B-G, Akira S, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature. 2008;456:264–8. https://doi.org/10.1038/nature07383.
Williams-Bey Y, Boularan C, Vural A, Huang N-N, Hwang I-Y, Shan-Shi C, Kehrl JH. Omega-3 Free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE. 2014;9(6):e97957. https://doi.org/10.1371/journal.pone.0097957.
Kawano A, Ariyoshi W, Yoshioka Y, Hikiji H, Nishihara T, Okinaga T. Docosahexaenoic acid enhances M2 macrophage polarization via the p38 signaling pathway and autophagy. J Cell Biochem. 2019;120:12604–17. https://doi.org/10.1002/jcb.28527.
Jessop F, Hamilton RF, Rhoderick JF, Shaw PK, Holian A. Autophagy deficiency in macrophages enhances NLRP3 inflammasome activity and chronic lung disease following silica exposure. Toxicol Appl Pharmacol. 2016;309:101–10. https://doi.org/10.1016/j.taap.2016.08.029.
Acknowledgements
The authors would like to thank the technical support from the CEHS Core Facilities: Inhalation and Pulmonary Physiology Core, Molecular Histology and Fluorescence Imaging Core, and the Fluorescence Cytometry Core. A special thank you to: Dr. Joanna Kreitinger at Dermaxon and Dr. Sarjubhai Patel at FYR Diagnostics for use of their 384-well CFX Maestro’s; Lou Herritt, and Pamela Shaw within the CEHS Core facilities; UM’s Laboratory Animal Resources technicians and facility; and Iheanyi Amadi for help with lung airway thickness analysis.
Funding
Paige Fletcher was supported by the Ruth L. Kirschstein NRSA Pre-doctoral Fellowship from the National Institute of Environmental Health Sciences (F31 ES028100). This research was supported by grants from the National Institute of Environmental Health Sciences (R01 ES023209 and R01 ES027353) and National Institute of General Medical Sciences (P30 GM103338). The content within is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Paige Fletcher was awarded one of QIAGEN’s featured young scientists of the month (November 2018) where she received QIAGEN products that contributed to this research.
Author information
Authors and Affiliations
Contributions
PF designed and carried out the in vivo studies, analyzed the in vivo and ex vivo studies, and performed statistical analysis. PF wrote the first draft of the manuscript. RFH setup the ex vivo studies, assisted in lung pathology scoring, and provided statistical advice. JFR assisted with mRNA quantification by qPCR and provided qPCR advice. BP and MB assisted PF with the in vivo studies. JJP assisted PF with logistics of the in vivo studies and supplied the DHA microalgal oil within the diets. AH assisted PF with overall study design and coordination. All authors contributed to furthering the manuscript’s drafts and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or competing interests to declare.
Ethical approval
The animal use protocol (035-16AHCEHS-062816) was approved by the University of Montana Institutional Animal Care and Use Committee for all mouse studies described within this manuscript. The mice are maintained in microisolation containers within the BSL-2 Laboratory Animal Resources facility at the University of Montana in the accordance with the Guide for the Care and Use of Laboratory Animals. The animal care facility at the University of Montana is staffed with full-time veterinarians that are AAALAC accredited. Mice were monitored on a daily basis along with during/after exposure to particles. Mice were anesthetized with isoflurane before particle or vehicle control instillations so as not to use any restraints or cause distress. All procedures within these studies caused minimal discomfort to the mice; however, in any cases where it was deemed that the mice were in pain or distress (adverse body weight, abnormal activity, poor grooming, abnormal posture) the animal was humanely euthanized.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fletcher, P., Hamilton, R.F., Rhoderick, J.F. et al. Therapeutic treatment of dietary docosahexaenoic acid for particle-induced pulmonary inflammation in Balb/c mice. Inflamm. Res. 70, 359–373 (2021). https://doi.org/10.1007/s00011-021-01443-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01443-4