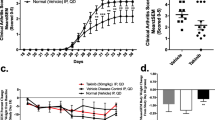
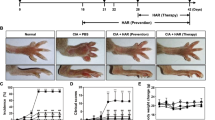
Abstract
Objective and design
To investigate the amelioration effects of quetiapine on rheumatoid arthritis with RAW 264.7 macrophage and collagen-induced arthritis (CIA) DBA/1J mouse model.
Subjects
RAW 264.7 macrophage and DBA/1J mice.
Treatment
Lipopolysaccharide and collagen.
Methods
RAW 264.7 macrophages stimulated by lipopolysaccharide (LPS) followed by quetiapine treatments were investigated. Activations of CD80 and CD86 were analyzed by flow cytometry. Pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β were analyzed by ELISA. Proteins involved in signaling pathways related to the formation of rheumatoid arthritis were assayed by Western blotting. Therapeutic efficacy of quetiapine in CIA mouse model was also assayed. 18F-FDG/micro-PET was used to monitor the inflammation status in the joints, and the severity of bone erosion was evaluated with micro-CT and H&E staining.
Results
The inhibition of pro-inflammatory cytokines by quetiapine was found through the ERK and AKT phosphorylation and subsequent NF-κB and CREB signaling pathways. Pro-inflammatory cytokines such as IL-17, IL-6 and IL-1β were decreased, while immunosuppressive factors such as TGF-β and IL-10 were increased in CIA mice treated with quetiapine. Notably, no uptake of 18F-FDG and bone erosion was found with micro-PET images on days 32 and 43 in the quetiapine-treated and normal control groups. However, significant uptake of 18F-FDG could be observed in the CIA group during the same time course. Similar results were further verified with ex vivo autoradiography.
Conclusion
Taken together, these results suggest that quetiapine is a potential anti-inflammatory drug, and may be used as an adjuvant for the treatment of rheumatoid arthritis.






Similar content being viewed by others
References
McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42.
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16.
Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403.
van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;5:531–41.
Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. https://doi.org/10.1101/cshperspect.a001651.
Dickens C, Creed F. The burden of depression in patients with rheumatoid arthritis. Rheumatology. 2001;40:1327–30.
Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42:233–46.
Bianchi M, Panerai AE. Antidepressant drugs and experimental inflammation. Pharmacol Res. 1996;33:235–38.
Baune BT, Eyre H. Anti-inflammatory effects of antidepressant and atypical antipsychotic medication for the treatment of major depression and comorbid arthritis: a case report. J Med Case Rep. 2010;4:6.
Kim H, Bang J, Chang HW, et al. Anti-inflammatory effect of quetiapine on collagen-induced arthritis of mouse. Eur J Pharmacol. 2012;678:55–60.
Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–16.
Rosloniec EF, Cremer M, Kang A, et al. Collagen-induced arthritis. Curr Protoc Immunol. 2010:89(1):15.5.1–15.5.25. https://doi.org/10.1002/0471142735.im1505s89
Wang WH, Chuang HY, Chen CH, et al. Lupeol acetate ameliorates collagen-induced arthritis and osteoclastogenesis of mice through improvement of microenvironment. Biomed Phamacother. 2016;79:231–40.
Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–75.
Tu SH, Chen YC, Hsiao TC, et al. Development of LabVIEW based micro computed tomography system on vertical rotary gantry. In: 6th European Conference of the International Federation for Medical and Biological Engineering. Lacković I, Vasic D, Springer International Publishing. 2015. Vol. 45, pp. 273–6.
Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1:612–9.
Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatol (Oxf). 2008;47:584–90.
Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–9.
Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–65.
Hsing CH, Lin MC, Choi PC, et al. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated AKT/IKKbeta/NF-kappaB signaling. PLoS One. 2011;6:e17598.
Eliopoulo AG, Dumitru CD, Wang CC, et al. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–40.
Du Y, Teng X, Wang N, et al. NF-κB and enhancer-binding CREB protein scaffolded by CREB-binding protein (CBP/p300) proteins regulate CD59 protein expression to protect cells from complement attack. J Biol Chem. 2014;289:2711–24.
Zhao Q, Wang X, Liu Y, et al. NFATc1: Functions in osteoclasts. Int J Biochem Cell B. 2010;42:576–9.
Beckers C, Ribbens C, André B, et al. Assessment of disease activity in rheumatoid arthritis with 18F-FDG PET. J Nucl Med. 2004;45:956–64.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76.
Kimura A, Kishimoto T. IL-6: regulator of TReg/Th17 balance. Eur J Immunol. 2010;40:1830–35.
Moon YM, Yoon BY, Her YM, et al. IL-32 and IL-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2012;14(6):R246. https://doi.org/10.1186/ar4089.
Arend WP. The mode of action of cytokine inhibitors. J Rheumatol Suppl. 2002;65:16–21.
Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104.
Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–12.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Hughes DE, Dai A, Tiffee JC, et al. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2:1132–6.
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49.
Wang K, Niu J, Kim H, et al. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J Mol Cell Biol. 2011;3:360–8.
Boivin WA, Cooper DM, Hiebert PR, et al. Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest. 2009;89:1195–220.
Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther 2006;8(Suppl 1):S3. https://doi.org/10.1186/ar1908.
Tajima T, Murata T, Aritake K, et al. Lipopolysaccharide induces macrophage migration via prostaglandin D2 and prostaglandin E2. J Pharmacol Exp Ther. 2008;326:493–501.
Maruotti N, Annese T, Cantatore FP, et al. Macrophages and angiogenesis in rheumatic diseases. Vasc Cell. 2013;5:11.
Craxton A, Magaletti D, Ryan EJ, et al. Macrophage- and dendritic cell–dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–71.
Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:566–71.
Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–75.
Munari F, FassAn M, Capitani N, et al. Cytokine BAFF released by Helicobacter pylori-infected macrophages triggers the Th17 response in human chronic gastritis. J Immunol. 2014;193:5584–94.
Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–86.
Nigrovic PA, Lee DM. Mast cells in inflammatory arthritis. Arthritis Res Ther. 2005;7:1–11.
Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13:235. https://doi.org/10.1186/ar3380.
Xiao S, Jin H, Korn T, et al. Retinoic acid increases Foxp3 + regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–84.
Kasama T, Strieter RM, Lukacs NW, et al. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995;95:2868–76.
Benson JM, Sachs CW, Treacy G, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29:615–24.
Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69.
Acknowledgements
We thank Mr. Yen-Po Lee for carrying out the experiments of infiltration of pro-inflammatory macrophages, neutrophils and differentiation of osteoclasts. This study was supported by a grant (105FN19) from Far Eastern Memorial Hospital-National Yang-Ming University Joint Research Program. We thank Taiwan Mouse Clinic (MOST 103-2325-B-001-015) which is funded by the National Research Program for Biophamaceuticals (NRPB) at the Ministry of Science and Technology (MOST) of Taiwan for the technical support of animal imaging facilities; and Biophotonics & Molecular Imaging Research Center (BMIRC), National Yang-Ming University, Taipei, Taiwan.
Author information
Authors and Affiliations
Contributions
YJP, WHW, CKF and JJH have contributed to the conception and design of the study and approved the final version of manuscript. TYH and YCC developed methods and performed experiments. TYH and WHW analyzed data and drafted the manuscript. All authors have contributed to interpretation of results, as well as contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Responsible Editor: Yoshiya Tanaka.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, YJ., Wang, WH., Huang, TY. et al. Quetiapine ameliorates collagen-induced arthritis in mice via the suppression of the AKT and ERK signaling pathways. Inflamm. Res. 67, 847–861 (2018). https://doi.org/10.1007/s00011-018-1176-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1176-1