Abstract
Objective
Glucagon-like peptide-1 (GLP-1)-based therapy via G protein-coupled receptor (GPCR) GLP-1R, to attenuate hyperglycemia in critical care has attracted great attention. However, the exaggerated inflammation by GLP-1R agonist, Exendin-4, in a mouse model of burn injury was quite unexpected. Recent studies found that GPCR might elicit proinflammatory effects by switching from Gαs to Gαi signaling in the immune system. Thus, we aimed to investigate the possible Gαs to Gαi switch in GLP-1R signaling in monocyte following burn injury.
Materials and methods
Splenic monocytes from sham and burn mice 24 h following burn injury were treated with consecutive doses of Exendin-4 alone or in combination with an inhibitor of Gαi signaling (pertussis toxin, PTX), or a blocker of protein kinase A (H89). Cell viability was assessed by CCK-8, and the supernatant was collected for cytokine measurement by ELISA. Intracellular cAMP level, phosphorylated PKA activity, and nuclear NF-κB p65 were determined by ELISA, ERK1/2 activation was analyzed by Western blot. The expression of GLP-1R downstream molecules, Gαs, Gαi and G-protein coupled receptor kinase 2 (GRK2) were examined by immunofluorescence staining and Western blot.
Results
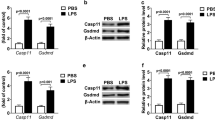
Exendin-4 could inhibit the viability of monocyte from sham rather than burn mice. Unexpectedly, it could also reduce TNF-α secretion from sham monocyte while increase it from burn monocyte. The increased secretion of TNF-α by Exendin-4 from burn monocyte could be reversed by pretreatment of PTX or H89. Accordingly, Exendin-4 could stimulates cAMP production dose dependently from sham instead of burn monocyte. However, the blunt cAMP production from burn monocyte was further suppressed by pretreatment of PTX or H89 after 6-h incubation. Nevertheless, phosphorylated PKA activity was significantly increased by low dose of Exendin-4 in sham monocyte, by contrast, it was enhanced by high dose of Exendin-4 in burn monocyte after 1-h incubation. Following Exendin-4 treatment for 2 h ex vivo, total nuclear NF-κB and phosphorylated NF-κB activity, as well as cytoplasmic pERK1/2 expressions were reduced in sham monocyte, however, only pERK1/2 was increased by Exendin-4 in burn monocytes. Moreover, reduced expressions of GLP-1R, GRK-2 and Gαs in contrast with increased expression of Gαi were identified in burn monocyte relative to sham monocyte.
Conclusions
This study presents an unexpected proinflammatory switch from Gαs to Gαi signaling in burn monocyte, which promotes ERK1/2 and NF-κB activation and the downstream TNF-α secretion. This phenomenon is most probably responsible for proinflammatory response evoked by Gαs agonist Exendin-4 following burn injury.






Similar content being viewed by others
References
Pinelli NR, Jones MC, Monday LM, Smith Z, Rhoney DH. Exogenous glucagon-like peptide-1 for hyperglycemia in critically ill patients. Ann Pharmacother. 2012;46:124–9.
Lebherz C, Schlieper G, Mollmann J, Kahles F, Schwarz M, Brunsing J, et al. GLP-1 levels predict mortality in patients with critical illness as well as end-stage renal disease. Am J Med. 2017;130:833–41 e3.
Kahles F, Meyer C, Mollmann J, Diebold S, Findeisen HM, Lebherz C, et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. 2014;63:3221–9.
Yanay O, Bailey AL, Kernan K, Zimmerman JJ, Osborne WR. Effects of exendin-4, a glucagon like peptide-1 receptor agonist, on neutrophil count and inflammatory cytokines in a rat model of endotoxemia. J Inflamm Res 2015;8:129–35.
Wang Y, Parlevliet ET, Geerling JJ, van der Tuin SJ, Zhang H, Bieghs V, et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br J Pharmacol. 2014;171:723–34.
Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–7.
Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64:2537–49.
Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425:304–8.
Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, et al. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–40.
Magocsi M, Vizi ES, Selmeczy Z, Brozik A, Szelenyi J. Multiple G-protein-coupling specificity of beta-adrenoceptor in macrophages. Immunology. 2007;122:503–13.
Weston C, Poyner D, Patel V, Dowell S, Ladds G. Investigating G protein signalling bias at the glucagon-like peptide-1 receptor in yeast. Br J Pharmacol. 2014;171:3651–65.
Shirazi R, Palsdottir V, Collander J, Anesten F, Vogel H, Langlet F, et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc Natl Acad Sci USA. 2013;110:16199–204.
Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–7.
Nielsen ST, Krogh-Madsen R, Moller K. Glucose metabolism in critically ill patients: are incretins an important player? J Intensive Care Med. 2015;30:201–8.
Watada S, Yu YM, Fischman AJ, Kurihara T, Shen CA, Tompkins RG, et al. Evaluation of intragastric vs intraperitoneal glucose tolerance tests in the evaluation of insulin resistance in a rodent model of burn injury and glucagon-like polypeptide-1 treatment. J Burn Care Res. 2014;35:e66–72.
Shen CA, Fagan S, Fischman AJ, Carter EE, Chai JK, Lu XM, et al. Effects of glucagon-like peptide 1 on glycemia control and its metabolic consequence after severe thermal injury—studies in an animal model. Surgery 2011;149:635–44.
Meier JJ, Weyhe D, Michaely M, Senkal M, Zumtobel V, Nauck MA, et al. Intravenous glucagon-like peptide 1 normalizes blood glucose after major surgery in patients with type 2 diabetes. Crit Care Med 2004;32:848–51.
Guo Z, Kavanagh E, Zang Y, Dolan SM, Kriynovich SJ, Mannick JA, et al. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171:3983–90.
Xiu F, Stanojcic M, Wang V, Qi P, Jeschke MG. C–C chemokine receptor type 2 expression on monocytes before sepsis onset is higher than that of postsepsis in septic burned patients: a new predictor for sepsis in burned injury. Ann Surg. 2016;264:392–8.
Noel G, Guo X, Wang Q, Schwemberger S, Byrum D, Ogle C. Postburn monocytes are the major producers of TNF-alpha in the heterogeneous splenic macrophage population. Shock. 2007;27:312–9.
Wu LL, Dong LW, Liu MS. Alterations of G-protein and adenylate cyclase signaling in rat liver during the progression of sepsis. Shock. 1999;11:39–43.
Wu LL, Yang SL, Yang RC, Hsu HK, Hsu C, Dong LW, et al. G protein and adenylate cyclase complex-mediated signal transduction in the rat heart during sepsis. Shock. 2003;19:533–7.
Link A, Selejan S, Maack C, Lenz M, Bohm M. Phosphodiesterase 4 inhibition but not beta-adrenergic stimulation suppresses tumor necrosis factor-alpha release in peripheral blood mononuclear cells in septic shock. Crit Care. 2008;12:R159.
Zhang QH, Chen Q, Kang JR, Liu C, Dong N, Zhu XM, et al. Treatment with gelsolin reduces brain inflammation and apoptotic signaling in mice following thermal injury. J Neuroinflamm. 2011;8:118.
Alexander M, Daniel T, Chaudry IH, Schwacha MG. Opiate analgesics contribute to the development of post-injury immunosuppression. J Surg Res. 2005;129:161–8.
Lai AY, Watanabe A, O’Brien T, Kondo M. Pertussis toxin-sensitive G proteins regulate lymphoid lineage specification in multipotent hematopoietic progenitors. Blood. 2009;113:5757–64.
Lee J, Kim TH, Murray F, Li X, Choi SS, Broide DH, et al. Cyclic AMP concentrations in dendritic cells induce and regulate Th2 immunity and allergic asthma. Proc Natl Acad Sci USA. 2015;112:1529–34.
Foey AD, Field S, Ahmed S, Jain A, Feldmann M, Brennan FM, et al. Impact of VIP and cAMP on the regulation of TNF-alpha and IL-10 production: implications for rheumatoid arthritis. Arthritis Res Ther. 2003;5:R317–28.
Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–9.
Jenei-Lanzl Z, Zwingenberg J, Lowin T, Anders S, Straub RH. Proinflammatory receptor switch from Galphas to Galphai signaling by beta-arrestin-mediated PDE4 recruitment in mixed RA synovial cells. Brain Behav Immun 2015;50:266–74.
Meisel C, Vogt K, Platzer C, Randow F, Liebenthal C, Volk HD. Differential regulation of monocytic tumor necrosis factor-alpha and interleukin-10 expression. Eur J Immunol. 1996;26:1580–6.
Kambayashi T, Jacob CO, Zhou D, Mazurek N, Fong M, Strassmann G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J Immunol. 1995;155:4909–16.
Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, et al. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA. 2003;100:940–5.
Stanojcic M, Chen P, Harrison RA, Wang V, Antonyshyn J, Zuniga-Pflucker JC, et al. Leukocyte infiltration and activation of the NLRP3 inflammasome in white adipose tissue following thermal injury. Crit Care Med. 2014;42:1357–64.
Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, et al. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock. 2007;28:684–93.
Vairo G, Argyriou S, Bordun AM, Whitty G, Hamilton JA. Inhibition of the signaling pathways for macrophage proliferation by cyclic AMP. Lack of effect on early responses to colony stimulating factor-1. J Biol Chem. 1990;265:2692–701.
Harikumar KG, Wootten D, Pinon DI, Koole C, Ball AM, Furness SG, et al. Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signaling but does not affect small molecule allostery. Proc Natl Acad Sci USA. 2012;109:18607–12.
Mittra S, Bourreau JP. Gs and Gi coupling of adrenomedullin in adult rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H1842–7.
Fan H, Li P, Zingarelli B, Borg K, Halushka PV, Birnbaumer L, et al. Heterotrimeric Galpha(i) proteins are regulated by lipopolysaccharide and are anti-inflammatory in endotoxemia and polymicrobial sepsis. Biochim Biophys Acta. 2011;1813:466–72.
Li P, Neubig RR, Zingarelli B, Borg K, Halushka PV, Cook JA, et al. Toll-like receptor-induced inflammatory cytokines are suppressed by gain of function or overexpression of Galpha(i2) protein. Inflammation. 2012;35:1611–7.
Hotta K, Hirshman CA, Emala CW. TNF-alpha increases transcription of Galpha(i-2) in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L319–25.
Reithmann C, Gierschik P, Werdan K, Jakobs KH. Tumor necrosis factor alpha up-regulates Gi alpha and G beta proteins and adenylyl cyclase responsiveness in rat cardiomyocytes. Eur J Pharmacol. 1991;206:53–60.
Hagen SA, Kondyra AL, Grocott HP, El-Moalem H, Bainbridge D, Mathew JP, et al. Cardiopulmonary bypass decreases G protein-coupled receptor kinase activity and expression in human peripheral blood mononuclear cells. Anesthesiology. 2003;98:343–8.
Solomon KR, Kurt-Jones EA, Saladino RA, Stack AM, Dunn IF, Ferretti M, et al. Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. J Clin Investig. 1998;102:2019–27.
Lentschat A, Karahashi H, Michelsen KS, Thomas LS, Zhang W, Vogel SN, et al. Mastoparan, a G protein agonist peptide, differentially modulates TLR4- and TLR2-mediated signaling in human endothelial cells and murine macrophages. J Immunol. 2005;174:4252–61.
Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled GI protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NFkappaB activation. Shock. 2004;22:57–62.
Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 2002;62:971–4.
Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91.
Park JY, Juhnn YS. cAMP signaling increases histone deacetylase 8 expression via the Epac2-Rap1A-Akt pathway in H1299 lung cancer cells. Exp Mol Med. 2017;49:e297.
Mirsaidi A, Tiaden AN, Richards PJ. Prostaglandin E2 inhibits matrix mineralization by human bone marrow stromal cell-derived osteoblasts via Epac-dependent cAMP signaling. Sci Rep. 2017;7:2243.
Thompson A, Kanamarlapudi V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Galphaq pathway. Biochem Pharmacol. 2015;93:72–84.
Roed SN, Wismann P, Underwood CR, Kulahin N, Iversen H, Cappelen KA, et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol. 2014;382:938–49.
Parvataneni S, Gonipeta B, Packiriswamy N, Lee T, Durairaj H, Parameswaran N. Role of myeloid-specific G-protein coupled receptor kinase-2 in sepsis. Int J Clin Exp Med. 2011;4:320–30.
Patial S, Saini Y, Parvataneni S, Appledorn DM, Dorn GW 2nd, Lapres JJ, et al. Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. J Cell Physiol. 2011;226:627–37.
Acknowledgements
This study was supported by National Natural Science Foundation (81272089), the National Basic Research Program of China (2012 CB518102), and Twelve-Five Plan for Military Scientific Foundation (BWS12J050).
Author information
Authors and Affiliations
Contributions
QHZ: development of the concept, conduction of most experiments, generation of all the data and figures, drafting and final approval the paper. JWH, GLL, XJJ and XDY: conduct part of the experiments. YMY: providing part of the fund and the personnel.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Zhang, QH., Hao, JW., Li, GL. et al. Proinflammatory switch from Gαs to Gαi signaling by Glucagon-like peptide-1 receptor in murine splenic monocyte following burn injury. Inflamm. Res. 67, 157–168 (2018). https://doi.org/10.1007/s00011-017-1104-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1104-9