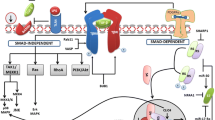
Abstract
Objective
The aims of this review are to describe the present knowledge about YKL-40 protein, discuss its relation to liver fibrosis, and to look ahead at future perspectives of YKL-40 research.
Introduction
Liver fibrosis is characterized by excess collagen deposition, decreased extracellular matrix degradation and activation of hepatic stellate cells. Therefore, advancement in the identification of liver fibrosis biomarkers with diagnostic and prognostic values becomes an important tool for future molecular therapy. The molecular basis of YKL-40 in liver fibrosis is unknown.
Methods
A PubMed database search was performed for studies of YKL-40 in liver injury and fibrosis.
Results and conclusions
YKL-40 is an inflammatory glycoprotein involved in endothelial dysfunction by promoting chemotaxis, cell attachment and migration, reorganization, and tissue remodeling as a response to endothelial damage. Several studies demonstrate that elevated serum YKL-levels are independently associated with the presence of endothelial damage and even higher YKL-40 levels are documented in liver fibrosis. YKL-40 may play a key role in liver injury and fibrosis.

Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- α-SMA:
-
α-smooth muscle actin
- HSC:
-
Hepatic stellate cells
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
References
Chiang DJ, Roychowdhury S, Bush K, McMullen MR, Pisano S, Niese K, et al. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS One. 2013;8:e69114.
Catanzaro R, Sapienza C, Milazzo M, Arona S, Italia A, Samperi L. Liver fibrosis: evaluation with diffusion-weighted magnetic resonance imaging in patients with chronic liver disease. Minerva Gastroenterol Dietol. 2013;59:313–20.
Lee JH, Kim JC, Tae G, Oh MK, Ko DK. Rapid diagnosis of liver fibrosis using multimodal multiphoton nonlinear optical microspectroscopy imaging. J Biomed Opt. 2013;18:76009.
Puche JE, Saiman Y, Friedman SL (2013) Hepatic stellate cells and liver fibrosis. Compr Physiol 3:1473–1492.
Fuchs BC, Wang H, Yang Y, Wei L, Polasek M, Schuhle DT, et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. J Hepatol. 2013;59(5):992–8.
Delgado MG, Gracia-Sancho J, Marrone G, Rodriguez-Vilarrupla A, Deulofeu R, Abraldes JG, et al. Leptin receptor blockade reduces intrahepatic vascular resistance and portal pressure in an experimental model of rat liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2013;305(7):G496–502.
Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int Off J Int Assoc Study Liver. 2013;33(10):1457–69.
Fuster D, Tsui JI, Cheng DM, Quinn EK, Armah KA, Nunes D, et al. Interleukin-6 is associated with noninvasive markers of liver fibrosis in hiv-infected patients with alcohol problems. AIDS Res Hum Retrovir. 2013;29:1110–6.
Abdalla AF, Fathy A, Zalata KR, Megahed A, Abo-Alyazeed A, El Regal ME. Morphometric assessment of liver fibrosis may enhance early diagnosis of biliary atresia. World J Pediatrics WJP. 2013;9(4):330–5.
Kornblit B, Hellemann D, Munthe-Fog L, Bonde J, Strom JJ, Madsen HO, et al. Plasma YKL-40 and CHI3L1 in systemic inflammation and sepsis-experience from two prospective cohorts. Immunobiology. 2013;218(10):1227–34.
Konradsen JR, James A, Nordlund B, Reinius LE, Soderhall C, Melen E, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013;132(2):328–35.
Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330.
Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One. 2013;8:e68780.
Jagavelu K, Routray C, Shergill U, O’Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590–601.
Marra F, Aleffi S, Galastri S, Provenzano A. Mononuclear cells in liver fibrosis. Semin Immunopathol. 2009;31:345–58.
Yang JJ, Tao H, Huang C, Li J. Nuclear erythroid 2-related factor 2: a novel potential therapeutic target for liver fibrosis. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2013;59C:421–7.
Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–13.
Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2013. doi:10.1002/hep.26604.
Nevzorova YA, Hu W, Cubero FJ, Haas U, Freimuth J, Tacke F, et al. Overexpression of c-myc in hepatocytes promotes activation of hepatic stellate cells and facilitates the onset of liver fibrosis. Biochim Biophys Acta. 2013;1832:1765–75.
Osawa Y, Hoshi M, Yasuda I, Saibara T, Moriwaki H, Kozawa O. Tumor necrosis factor-alpha promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS One. 2013;8:e65251.
Saito S, Hata K, Iwaisako K, Yanagida A, Takeiri M, Tanaka H, et al. Cilostazol attenuates hepatic stellate cell activation and protects mice against carbon tetrachloride-induced liver fibrosis. Hepatol Res Off J Jpn Soc Hepatol. 2013. doi:10.1111/hepr.12140.
Yang L, Stimpson SA, Chen L, Wallace Harrington W, Rockey DC, et al. Effectiveness of the PPARgamma agonist, GW570, in liver fibrosis. Inflamm Res Off J Eur Hist Res Soc. 2010;59:1061–71.
Novo E, Parola M. The role of redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S4.
Lv Z, Song Y, Xue D, Zhang W, Cheng Y, Xu L. Effect of salvianolic-acid B on inhibiting MAPK signaling induced by transforming growth factor-beta1 in activated rat hepatic stellate cells. J Ethnopharmacol. 2010;132:384–92.
Li J, Li S, He B, Mi Y, Cao H, Zhang C, et al. Ameliorative effect of grape seed proanthocyanidin extract on thioacetamide-induced mouse hepatic fibrosis. Toxicol Lett. 2012;213:353–60.
Klein S, Klosel J, Schierwagen R, Korner C, Granzow M, Huss S, et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Investig J Tech Methods Pathol. 2012;92:1440–50.
Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, et al. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complim Altern Med. 2012;12:51.
Bieghs V, Walenbergh SM, Hendrikx T, van Gorp PJ, Verheyen F, Olde Damink SW, et al. Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver Int Off J Int Assoc Study Liver. 2013;33:1056–61.
Su LJ, Chang CC, Yang CH, Hsieh SJ, Wu YC, Lai JM, et al. Graptopetalum paraguayense ameliorates chemical-induced rat hepatic fibrosis in vivo and inactivates stellate cells and Kupffer cells in vitro. PLoS One. 2013;8:e53988.
Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Investig J Tech Methods Pathol. 2003;83:163–73.
Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–23.
Harutyunyan M, Christiansen M, Johansen JS, Kober L, Torp-Petersen C, Kastrup J. The inflammatory biomarker YKL-40 as a new prognostic marker for all-cause mortality in patients with heart failure. Immunobiology. 2012;217:652–6.
Nielsen KR, Steffensen R, Boegsted M, Baech J, Lundbye-Christensen S, Hetland ML, et al. Promoter polymorphisms in the chitinase 3-like 1 gene influence the serum concentration of YKL-40 in Danish patients with rheumatoid arthritis and in healthy subjects. Arthritis Res Ther. 2011;13:R109.
Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32:949–55.
Schimpl M, Rush CL, Betou M, Eggleston IM, Recklies AD, van Aalten DM. Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem J. 2012;446:149–57.
Roslind A, Johansen JS. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol Biol. 2009;511:159–84.
Areshkov PO, Avdieiev SS, Balynska OV, Leroith D, Kavsan VM. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different influence on cell proliferation. Int J Biol Sci. 2012;8:39–48.
Scully S, Yan W, Bentley B, Cao QJ, Shao R. Inhibitory activity of YKL-40 in mammary epithelial cell differentiation and polarization induced by lactogenic hormones: a role in mammary tissue involution. PLoS One. 2011;6:e25819.
Prakash M, Bodas M, Prakash D, Nawani N, Khetmalas M, Mandal A, et al. Diverse pathological implications of YKL-40: answers may lie in ‘outside-in’ signaling. Cell Signal. 2013;25:1567–73.
Tache D, Bogdan F, Pisoschi C, Banita M, Stanciulescu C, Fusaru AM, et al. Evidence for the involvement of TGF-beta1-CTGF axis in liver fibrogenesis secondary to hepatic viral infection. Rom J Morphol Embryol. 2011;52:409–12.
Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Investig J Tech Methods Pathol. 2007;87:858–70.
Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003;278:44058–67.
Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PE. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005;96:183–90.
Antonelli M, Massimino M, Morra I, Garre ML, Gardiman MP, Buttarelli FR, et al. Expression of pERK and pAKT in pediatric high grade astrocytomas: correlation with YKL40 and prognostic significance. Neuropathol Off J Jpn Soc Neuropathol. 2012;32:133–8.
Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:3935–41.
Gonzalez-Fernandez O, Jimenez A, Villalobo A. Differential p38 mitogen-activated protein kinase-controlled hypophosphorylation of the retinoblastoma protein induced by nitric oxide in neuroblastoma cells. Free Radic Biol Med. 2008;44:353–66.
Chrissouli S, Pratsinis H, Velissariou V, Anastasiou A, Kletsas D. Human amniotic fluid stimulates the proliferation of human fetal and adult skin fibroblasts: the roles of bFGF and PDGF and of the ERK and Akt signaling pathways. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2010;18:643–54.
Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol. 2013;190:5226–36.
Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–8.
Berres ML, Papen S, Pauels K, Schmitz P, Zaldivar MM, Hellerbrand C, et al. A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J Hepatol. 2009;50:370–6.
Lee CK, Perez-Atayde AR, Mitchell PD, Raza R, Afdhal NH, Jonas MM. Serum biomarkers and transient elastography as predictors of advanced liver fibrosis in a United States cohort: the Boston Children’s Hospital Experience. J Pediatr. 2013;163(4):1058–64.
Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis. 2013;13:203.
Eurich D, Neumann UP, Boas-Knoop S, Neuhaus R, Kiessling A, Yahyazadeh A, et al. YKL-40-gene polymorphism affects acute cellular rejection and fibrosis progression after transplantation for hepatitis C virus-induced liver disease. J Gastroenterol Hepatol. 2013;28:153–60.
Wang D, Lu JG, Wang Q, Du XL, Dong R, Wang P, et al. Increased immunohistochemical expression of YKL-40 in the spleen of patients with portal hypertension. Braz J Med Biol Research. 2012;45:264–72.
Lebensztejn DM, Wierzbicka A, Socha P, Pronicki M, Skiba E, Werpachowska I, et al. Cytokeratin-18 and hyaluronic acid levels predict liver fibrosis in children with non-alcoholic fatty liver disease. Acta Biochim Polonica. 2011;58:563–6.
Fontana RJ, Litman HJ, Dienstag JL, Bonkovsky HL, Su G, Sterling RK, et al. YKL-40 genetic polymorphisms and the risk of liver disease progression in patients with advanced fibrosis due to chronic hepatitis C. Liver Int Off J Int Assoc Study Liver. 2012;32:665–74.
Pizano-Martinez O, Yanez-Sanchez I, Alatorre-Carranza P, Miranda-Diaz A, Ortiz-Lazareno PC, Garcia-Iglesias T, et al. YKL-40 expression in CD14(+) liver cells in acute and chronic injury. World J Gastroenterol WJG. 2011;17:3830–5.
Rath T, Roderfeld M, Guler C, Wenzel C, Graf J, Beitinger F, et al. YKL-40 and transient elastography, a powerful team to assess hepatic fibrosis. Scand J Gastroenterol. 2011;46:1369–80.
Fontana RJ, Dienstag JL, Bonkovsky HL, Sterling RK, Naishadham D, Goodman ZD, et al. Serum fibrosis markers are associated with liver disease progression in non-responder patients with chronic hepatitis C. Gut. 2010;59:1401–9.
Schiavon LL, Carvalho-Filho RJ, Narciso-Schiavon JL, Medina-Pestana JO, Lanzoni VP, Ferraz ML, et al. YKL-40 and hyaluronic acid (HA) as noninvasive markers of liver fibrosis in kidney transplant patients with HCV chronic infection. Scand J Gastroenterol. 2010;45:615–22.
Fontana RJ, Bonkovsky HL, Naishadham D, Dienstag JL, Sterling RK, Lok AS, et al. Serum fibrosis marker levels decrease after successful antiviral treatment in chronic hepatitis C patients with advanced fibrosis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7:219–26.
Pungpapong S, Nunes DP, Krishna M, Nakhleh R, Chambers K, Ghabril M, et al. Serum fibrosis markers can predict rapid fibrosis progression after liver transplantation for hepatitis C. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2008;14:1294–302.
Mehta P, Ploutz-Snyder R, Nandi J, Rawlins SR, Sanderson SO, Levine RA. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2008;103:928–36.
Lebensztejn DM, Skiba E, Werpachowska I, Sobaniec-Lotowska ME, Kaczmarski M. Serum level of YKL-40 does not predict advanced liver fibrosis in children with chronic hepatitis B. Adv Med Sci. 2007;52:120–4.
Fontana RJ, Goodman ZD, Dienstag JL, Bonkovsky HL, Naishadham D, Sterling RK, et al. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology. 2008;47:789–98.
Esmat G, Metwally M, Zalata KR, Gadalla S, Abdel-Hamid M, Abouzied A, et al. Evaluation of serum biomarkers of fibrosis and injury in Egyptian patients with chronic hepatitis C. J Hepatol. 2007;46:620–7.
Kamal SM, Turner B, He Q, Rasenack J, Bianchi L, Al Tawil A, et al. Progression of fibrosis in hepatitis C with and without schistosomiasis: correlation with serum markers of fibrosis. Hepatology. 2006;43:771–9.
Zheng M, Cai WM, Zhao JK, Zhu SM, Liu RH. Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta Trop. 2005;96:148–52.
Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol WJG. 2005;11:476–81.
Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2006;15:194–202.
Acknowledgments
This project was supported by the National Science Foundation of China (NO 81273526, 81202978), and by the Anhui Provincial Natural Science Foundation (1308085MH117).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Kumar Visvanathan.
H. Tao and J.-J. Yang contribute equally to the first author.
Rights and permissions
About this article
Cite this article
Tao, H., Yang, JJ., Shi, KH. et al. The significance of YKL-40 protein in liver fibrosis. Inflamm. Res. 63, 249–254 (2014). https://doi.org/10.1007/s00011-013-0698-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0698-9