Abstract
Objectives and design
Arbutin, which is found in the genus Arctostaphylos, is an anti-oxidant and a depigmenting agent. The present study was designed to validate the anti-inflammatory effect of arbutin.
Materials and methods
The anti-inflammatory properties of arbutin were studied using a lipopolysaccharide (LPS)-stimulated murine BV2 microglial cells model. As inflammatory parameters, the production of nitric oxide (NO), inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6) were evaluated. We also examined the expression of ninjurin1 (Ninj1) and the adhesion activity of BV2 cells. Finally, we analyzed the activation of the nuclear factor-κB (NF-κB) signaling pathway.
Results
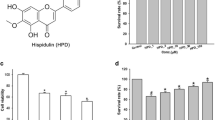
Arbutin suppressed LPS-induced production of NO and expression of iNOS and COX-2 in a dose-dependent manner without causing cellular toxicity. Arbutin also significantly reduced generation of proinflammatory cytokines, including IL-1β and TNF-α, and other inflammation-related genes such as MCP-1 and IL-6. Additionally, arbutin suppressed the adhesion activity of BV2 cells and the expression of an important adhesion molecule, Ninj1, in LPS-stimulated murine BV2 cells. Furthermore, arbutin inhibited nuclear translocation and the transcriptional activity of NF-κB.
Conclusions
Taken together, our results suggest that arbutin might be useful for treating the inflammatory and deleterious effects of BV2 microglial cells activation in response to LPS stimulation.






Similar content being viewed by others
References
Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–49.
Barin JG, Rose NR, Cihakova D. Macrophage diversity in cardiac inflammation: a review. Immunobiology. 2011;217:468–75.
Papageorgiou AP, Heymans S. Interactions between the extracellular matrix and inflammation during viral myocarditis. Immunobiology. 2011;217:503–10.
Chen YW, Pat B, Gladden JD, Zheng J, Powell P, Wei CC, et al. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol. 2011;300:H2251–60.
Kang MJ, Ha HW, Kim HG, Lee DH, Kong MJ, Ahn YT, et al. Role of metabolism by intestinal bacteria in arbutin-induced toxicity in vitro. Arch Pharm Res. 2011;34:687–93.
Zen JM, Yang HH, Chiu MH, Yang CH, Shih Y. Selective determination of arbutin in cosmetic products through online derivatization followed by disposable electrochemical sensor. J AOAC Int. 2011;94:985–90.
Hori I, Nihei K, Kubo I. Structural criteria for depigmenting mechanism of arbutin. Phytother Res. 2004;18:475–9.
Bang SH, Han SJ, Kim DH. Hydrolysis of arbutin to hydroquinone by human skin bacteria and its effect on antioxidant activity. J Cosmet Dermatol. 2008;7:189–93.
Pavlovic RD, Lakusic B, Doslov-Kokorus Z, Kovacevic N. Arbutin content and antioxidant activity of some Ericaceae species. Pharmazie. 2009;64:656–9.
Takebayashi J, Ishii R, Chen J, Matsumoto T, Ishimi Y, Tai A. Reassessment of antioxidant activity of arbutin: multifaceted evaluation using five antioxidant assay systems. Free Radic Res. 2010;44:473–8.
Cheng SL, Liu RH, Sheu JN, Chen ST, Sinchaikul S, Tsay GJ. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J Biomed Sci. 2007;14:87–105.
Funayama M, Arakawa H, Yamamoto R, Nishino T, Shin T, Murao S. Effects of alpha- and beta-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci Biotechnol Biochem. 1995;59:143–4.
Nawarak J, Huang-Liu R, Kao SH, Liao HH, Sinchaikul S, Chen ST, et al. Proteomics analysis of A375 human malignant melanoma cells in response to arbutin treatment. Biochim Biophys Acta. 2009;1794:159–67.
Sugimoto K, Nishimura T, Nomura K, Kuriki T. Inhibitory effects of alpha-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model. Biol Pharm Bull. 2004;27:510–4.
Callender VD, St Surin-Lord S, Davis EC, Maclin M. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87–99.
Lee HJ, Ahn BJ, Shin MW, Choi JH, Kim KW. Ninjurin1: a potential adhesion molecule and its role in inflammation and tissue remodeling. Mol Cells. 2010;29:223–7.
Ahn BJ, Lee HJ, Shin MW, Choi JH, Jeong JW, Kim KW. Ninjurin1 is expressed in myeloid cells and mediates endothelium adhesion in the brains of EAE rats. Biochem Biophys Res Commun. 2009;387:321–5.
Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009;16:1395–407.
Lee SH, Kim DW, Back SS, Hwang HS, Park EY, Kang TC, et al. Transduced Tat-Annexin protein suppresses inflammation-associated gene expression in lipopolysaccharide (LPS)-stimulated Raw 264.7 cells. BMB Rep. 2011;44:484–9.
Sachithanandan N, Graham KL, Galic S, Honeyman JE, Fynch SL, Hewitt KA, et al. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60:2023–31.
Lee H, Bae S, Choi BW, Yoon Y. WNT/beta-catenin pathway is modulated in asthma patients and LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol. Immunotoxicol. 2012;34:56–65.
Karpurapu M, Wang X, Deng J, Park H, Xiao L, Sadikot RT, et al. Functional PU.1 in macrophages has a pivotal role in NF-{kappa}B activation and neutrophilic lung inflammation during endotoxemia. Blood. 2011;118:5255–66.
Tucsek Z, Radnai B, Racz B, Debreceni B, Priber JK, Dolowschiak T, et al. Suppressing LPS-induced early signal transduction in macrophages by a polyphenol degradation product: a critical role of MKP-1. J Leukoc Biol. 2011;89:105–11.
Baowen Q, Yulin Z, Xin W, Wenjing X, Hao Z, Zhizhi C, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol. 2010;642:134–9.
Ifergan I, Kebir H, Terouz S, Alvarez JI, Lécuyer MA, Gendron S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70:751–63.
Hamed SH, Sriwiriyanont P, deLong MA, Visscher MO, Wickett RR, Boissy RE. Comparative efficacy and safety of deoxyarbutin, a new tyrosinase-inhibiting agent. J Cosmet Sci. 2006;57:291–308.
Choi MY, Song HS, Hur HS, Sim SS. Whitening activity of luteolin related to the inhibition of cAMP pathway in alpha-MSH-stimulated B16 melanoma cells. Arch Pharm Res. 2008;31:1166–71.
Xiao L, Matsubayashi K, Miwa N. Inhibitory effect of the water-soluble polymer-wrapped derivative of fullerene on UVA-induced melanogenesis via downregulation of tyrosinase expression in human melanocytes and skin tissues. Arch Dermatol Res. 2007;299:245–57.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) through the Creative Research Initiative Program (R16-2004-001-01001-0) and the Global Research Laboratory Program (2011-0021874).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Graham Wallace.
Rights and permissions
About this article
Cite this article
Lee, HJ., Kim, KW. Anti-inflammatory effects of arbutin in lipopolysaccharide-stimulated BV2 microglial cells. Inflamm. Res. 61, 817–825 (2012). https://doi.org/10.1007/s00011-012-0474-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0474-2