Abstract
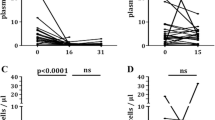
A Euro-Lupus regimen of low-dose intravenous cyclophosphamide (CFA) is commonly used to treat severe organ manifestations of systemic lupus erythematosus (SLE), particularly lupus nephritis (LN). There are no data on the distributions and dynamics of immune cell populations in patients with various treatment outcomes. The circulating immune cells of 11 female SLE patients were assessed before and after Euro-Lupus regimen (cumulative dose of 3000 mg CFA) by flow cytometry together with those of 16 healthy women. A subanalysis was performed in LN patients who achieved complete remission (CR; n = 3), partial remission (PR; n = 4), and no response (NR; n = 2). In SLE, the Euro-Lupus regimen decreased the percentage and absolute count of B cells; increased the percentage of CD8+ T cells, T regulatory cells, neutrophils, and monocyte subsets; and activated T and NK cells compared to healthy controls (P < 0.050). Patients with LN achieving CR had significantly lower proportions of CD27+ B memory cells compared to poor responders (PR/NR, P = 0.035). The post-treatment percentages and absolute numbers of B cells, T cells, NK cells, monocytes, and neutrophils showed high inter-individual variability with no association with treatment outcome. Our pilot study revealed the dynamics of changes in immune cell populations in SLE patients during a Euro-Lupus regimen, mainly the lowering of B cells. In LN patients who achieved CR, a lower proportion of CD27+ B memory cells was evident compared to poor responders (PR/NR). Further studies on usefulness of monitoring immune cells for treatment response prediction on larger cohorts are needed.



Similar content being viewed by others
References
Agematsu K, Nagumo H, Oguchi Y et al (1998) Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood 91:173–180
Ahlmann M, Hempel G (2016) The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 78:661–671
Alegretti AP, Schneider L, Piccoli AK et al (2012) diminished expression of complement regulatory proteins on peripheral blood cells from systemic lupus erythematosus patients. Clin Dev Immunol 2012:725684
Amano H, Morimoto S, Kaneko H et al (2000) Effect of intravenous cyclophosphamide in systemic lupus erythematosus: relation to lymphocyte subsets and activation markers. Lupus 9:26–32
Austin AH, Illei GG, Braun MJ et al (2009) Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 20:901–911
Boldt A, Kahlenberg F, Fricke S et al (2014) Flow cytometric phenotyping of lymphocytes in patients with systemic lupus erythematosus. Cytometry A 85:567–569
Carmona-Rivera C, Kaplan MJ (2014) Detection of SLE antigens in neutrophil extracellular traps (NETs). Methods Mol Biol 1134:151–161
Chen YE, Korbet SM, Katz RS et al (2008) Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 3:46–53
Costa N, Marques O, Godinho SI et al (2017) Two separate effects contribute to regulatory T cell defect in systemic lupus erythematosus patients and their unaffected relatives. Clin Exp Immunol 189:318–330
Cruz-González DJ, Gómez-Martin D, Layseca-Espinosa E et al (2018) Analysis of the regulatory function of natural killer cells from patients with systemic lupus erythematosus. Clin Exp Immunol 191:288–300
Dörner T, Jacobi AM, Lipsky PE (2009) B cells in autoimmunity. Arthritis Res Ther 11:247
Fanouriakis A, Pamfil C, Sidiropoulos P et al (2016) Cyclophosphamide in combination with glucocorticoids for severe neuropsychiatric systemic lupus erythematosus: a retrospective, observational two-centre study. Lupus 25:627–636
Fassbinder T, Saunders U, Mickholz E et al (2015) Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther 17:92
Gladman DD, Urowitz MB, Kagal A et al (2000) Accurately describing changes in disease activity in systemic lupus erythematosus. J Rheumatol 27:377–379
Gómez-Martín D, Díaz-Zamudio M, Vanoye G et al (2011) Quantitative and functional profiles of CD4+ lymphocyte subsets in systemic lupus erythematosus patients with lymphopenia. Clin Exp Immunol 164:17–25
Horák P, Tegzová D, Závada Z et al (2013) Recommendation of Czech Rheumatology Society for treatment of systemic lupus erythematosus [in Czech]. Čes Revmatol 21:110–122
Horwitz DA (2008) Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther 10:227
Houssiau FA, Vasconcelos C, D’Cruz D et al (2002) Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46:2121–2131
Houssiau FA, Vasconcelos C, D’Cruz D et al (2010) The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 69:61–64
Hui-Yuen JS, Nguyen SC, Askanase AD (2016) Targeted B cell therapies in the treatment of adult and pediatric systemic lupus erythematosus. Lupus 25:1086–1096
Jacobi AM, Reiter K, Mackay M et al (2008) Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 58:1762–1773
Kaplan MJ (2011) Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol 7:691–699
Katsuyama T, Tsokos GC, Moulton VR (2018) Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol 9:1088
Kawabata D, Venkatesh J, Ramanujam M et al (2010) Enhanced selection of high affinity DNA-reactive b cells following cyclophosphamide treatment in mice. PLoS One 5:e8418
Lacki JK, Mackiewicz SH, Leszczynski P et al (1997) The effect of intravenous cyclophosphamide pulse on peripheral blood lymphocytes in lupus erythematosus patients. Rheumatol Int 17:55–60
Lewis EE, McCune WJ, Knight JS (2016) Neutrophilia in systemic lupus erythematosus as a potential indicator of disease activity. Arthritis Rheumatol 2016(suppl 10):68
Liu Z, Davidson A (2012) Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 18:871–882
Manukyan G, Papajik T, Gajdos P et al (2017) Neutrophils in chronic lymphocytic leukemia are permanently activated and have functional defects. Oncotarget 8:84889–84901
Merrill JT, Buyon JP (2005) The role of biomarkers in the assessment of lupus. Best Pract Res Clin Rheumatol 19:709–726
Moulton VR, Tsokos GC (2015) T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest 125:2220–2227
Moulton VR, Suarez-Fueyo A, Meidan E et al (2017) Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol Med 23:615–635
Nashi E, Wang Y, Diamond B (2010) The role of B cells in lupus pathogenesis. Int J Biochem Cell Biol 42:543–550
Oaks Z, Winans T, Huang N et al (2016) Activation of the mechanistic target of rapamycin in SLE: explosion of evidence in the last five years. Curr Rheumatol Rep 18:73
Odendahl M, Jacobi A, Hansen A et al (2000) Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 165:5970–5979
Pan X, Yuan X, Zheng Y et al (2012) Increased CD45RA + FoxP3(low) regulatory T cells with impaired suppressive function in patients with systemic lupus erythematosus. PLoS One 7:e34662
Park YW, Kee SJ, Cho YN et al (2009) Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum 60:1753–1763
Perl A (2016) Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol 12:169–182
Ren Y, Tang J, Mok MY et al (2003) Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum 48:2888–2897
Shirakawa F, Yamashita U, Suzuki H (1985) Decrease in HLA-DR-positive monocytes in patients with systemic lupus erythematosus (SLE). J Immunol 134:3560–3562
Silva-Neta HL, Brelaz-de-Castro MCA, Chagas MBO et al (2018) CD4+ CD45RA-FOXP3low regulatory T cells as potential biomarkers of disease activity in systemic lupus erythematosus Brazilian patients. Biomed Res Int 2018:3419565
Smith CK, Kaplan MJ (2015) The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol 27:448–453
Steinbach F, Henke F, Krause B et al (2000) Monocytes from systemic lupus erythematous patients are severely altered in phenotype and lineage flexibility. Ann Rheum Dis 59:283–288
Tamirou F, Husson SN, Gruson D et al (2017) Brief Report: the euro-lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by serum levels of anti-müllerian hormone. Arthritis Rheumatol 69:1267–1271
Tan EM, Cohen AS, Fries JF et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Tselios K, Sarantopoulos A, Gkougkourelas I et al (2014) Increase of peripheral T regulatory cells during remission induction with cyclophosphamide in active systemic lupus erythematosus. Int J Rheum Dis 17:790–795
Tselios K, Sarantopoulos A, Gkougkourelas I et al (2015) The influence of therapy on CD4 + CD25(high)FOXP3 + regulatory T cells in systemic lupus erythematosus patients: a prospective study. Scand J Rheumatol 44:29–35
van Vollenhoven R, Voskuyl A, Bertsias G et al (2017) A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 76:554–561
Weening JJ, Dagati WD, Schwartz MM et al (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15:241–250
Wu Y, Chen Y, Yang X et al (2016) Neutrophil-to-lymphocyte ratio (NLR) and Platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol 36:94–99
Zhao L, Jiang Z, Jiang Y et al (2012) Changes in immune cell frequencies after cyclophosphamide or mycophenolate mofetil treatments in patients with systemic lupus erythematosus. Clin Rheumatol 31:951–959
Acknowledgements
We would like to thank Mr. Jakub Savara, for his kind help with figures.
Funding
This work was supported by the Internal Grant Agency of Palacký University (IGA_LF_2019_006, and IGA_LF_2019_014), and, in part, by the Ministry of Health of Czech Republic (MH CZ—DRO (FNOL, 00098892).
Author information
Authors and Affiliations
Contributions
GG, ZM, AP: laboratory measurement, GG, SZ: statistical analysis, GG, EK: writing of the manuscript and interpretation of data, PH, MS, AS: collection of clinical data, PH, FM, MZ: critical revision of the manuscript, EK, PH: study design.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Gabcova, G., Horak, P., Mikulkova, Z. et al. Modulatory Effect of the Euro-Lupus Low-Dose Intravenous Cyclophosphamide Regimen on Circulating Immune Cells in Systemic Lupus Erythematosus. Arch. Immunol. Ther. Exp. 67, 415–425 (2019). https://doi.org/10.1007/s00005-019-00563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-019-00563-4