Abstract
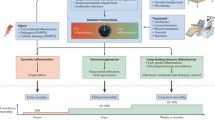
Septic syndromes are the main cause of death in the intensive care units and although the mortality rates is slowly decreasing, the occurrence of the disease has been increasing. The pathogenesis of sepsis includes countless disturbances of the host immune system starting with a harmful, infection-triggered exaggerated inflammatory cascade, followed by the development of an immunoparalysis state. The latter contributes to the failure in pathogen eradication and leads to secondary infections, which are often the cause of fatal complications. In this review, we consider different novel therapeutic strategies for restoration of immune function. The use of glucocorticoids, intravenous immunoglobulins, heparin, recombinant human activated protein C, granulocyte macrophage colony-stimulating factor, granulocyte colony-stimulating factor, interferon-γ, statins, macrolides and high-volume hemofiltration are discussed. Even though some clinical trials of these regimens are promising, the key to their successful application seems to be the precise monitoring of the status of immune system followed by implementation of the adequate therapy. Thus, in this paper we present disturbances in the immune system in the course of human sepsis, with special attention to the parameters that could be monitored and serve as markers for immunomodulatory therapies. We conclude by briefly presenting the current sepsis treatment strategy.
Similar content being viewed by others
Introduction
Sepsis, severe sepsis and septic shock still constitute a major and underestimated clinical problem. Despite the progress in basic science, both understanding of the pathomechanisms and the therapy of sepsis remains challenging. Septic syndromes are the number one cause of deaths in the intensive care units (ICU) in Europe and US. The mortality rates vary between 30 and 60%, due to a range of definitions applied in the reviews about sepsis and its complications (Angus and Linde-Zwirble 2001; Levy et al. 2010). Another reason for the broad range of estimates lies in the differences between studied populations. According to 131 studies on septic shock and conducted between 1958 and 1997, the overall mortality rate was 49.7%. About one-quarter of deaths can be classified as a complication of bacteremia while another 10% is directly caused by the disease itself. Growing invasiveness of medical procedures, expanding number of immunocompromised patients, massive use of broad-spectrum antibiotics and aging of societies are the main reasons for the increasing occurrence of sepsis currently estimated to be the cause of 1.5% of deaths each year. It was calculated that the diagnosis of sepsis is made in 1.8 million patients a year (Angus and Linde-Zwirble 2001; Levy et al. 2010).
In the early 1990s a strong connection between sepsis and the systemic inflammatory response syndrome (SIRS) was noted (Bone et al. 1992). Local inflammation is definitely a beneficial mechanism of the host defense response against infection. However, when it is followed by the massive release of proinflammatory mediators, such as tumor necrosis factor (TNF), interleukin 1β (IL-1β), IL-6, IL-12, anaphylotoxins, uncontrolled SIRS can be developed. This excessive inflammatory response was thought to be the main cause of death. Such deduction led to numerous trials to block the inflammatory cascade by high doses of glucocorticosteroids, neutralizing antibodies directed against endotoxin and early inflammatory cytokines such as TNF or IL-1. Although results of studies in animal models were encouraging, clinical trials failed to improve outcome of sepsis (Opal et al. 1997; Reinhart et al. 2001), which led to increasing doubts in the whole hypothesis (Bone 1996). Next, the attention of researchers was drawn by the observation that most deaths of sepsis patients occurs in the later phase of the disease, which is probably due to the development of secondary infection in the course of the multi-organ dysfunction syndrome. It is known from autopsy studies that most of the patients that died of sepsis had active sites of infection, in spite of intensive antimicrobial treatment (Torgersen et al. 2009). In fact, the immune response is a dynamic process disturbing the homeostatic mechanisms, rather than a simple state of hyperactivity. The primary activation of inflammatory pathways is followed by multi-organ dysfunction, hypotension and hypoperfusion, but it also activates opposite mechanisms, known as the compensatory anti-inflammatory response syndrome (Bone 1996). First, in order to restore the immune balance, this process operates as a part of homeostasis. Only later a form of immunosuppression, called “immunoparalysis” is evoked and is the probable reason for therapeutic failures. At present, many immunomodulating therapies are considered, but because of the dynamics of the host defense system, introduction of such regimens requires individual and precise analysis of the actual state of the patient immune system (Adib-Conquy and Cavaillon 2009; Draisma et al. 2008; Monneret et al. 2008). The possibilities of monitoring the immune system disturbances are discussed below and summarized in Table 1.
Immune System and Its Activation in Sepsis
Various microorganisms may be recognized by the cells of innate immune system because of their expression of the so called pathogen-associated molecular patterns (PAMPs), i.e. murein and lipopolysaccharide (LPS) in bacteria, mannans in fungi and double-strain RNA in some viruses. These PAMPs are recognized by pattern recognition receptors (PARRs), including Toll-like receptors (TLR). Activation of PARRs is the first step of activating immune response (Bone 1996; Draisma et al. 2008; Torgersen et al. 2009).
Mechanisms of the initiation of the inflammatory cascade are well studied. Numerous exogenous (endo- and exotoxin) and endogenous mediators, such as activated complement factor (C5a), active Hagemann factor (XIIa) or damaged cells, are capable of activating monocytes and macrophages, which synthesize cytokines responsible for the development of SIRS, like TNF, IL-1β, IL-6 and interferon γ (IFN-γ). The intracellular inflammatory pathway is commonly started by a non-specific oxidative stress in the following mechanism: exogenous stimuli (i.e. LPS) activate serine phosphokinase via ligation to PRR, which evokes oxidative reactions that in turn activate nuclear factor κB (NF-κB) by splitting off its inhibitor. The inhibitor of κB active dimer migrates to the nuclei, where it regulates transcription of genes for proinflammatory cytokines (TNF, IL-1β) and proinflammatory enzymes: inducible nitric oxide synthase (iNOS) and cyclooxygenase 2. The iNOS synthase is a key player in septic circulatory dysfunctions through both strong cardiodepressive effect and vasodilatatory effect in microcirculation (Adib-Conquy and Cavaillon 2009; Bone 1996; Draisma et al. 2008; Monneret et al. 2008).
Targets for Monitoring Immune Dysfunctions
Circulating Mediators
Monitoring the patient’s plasma concentration of proinflammatory cytokines is a relatively cheap and easy test, which therefore may be helpful in the introduction of immunostimulatory treatment (Schefold et al. 2008). Although huge efforts have been made, the concentration of no single inflammatory mediator has been identified as a reliable marker of the patient clinical state. This may seem quite obvious as hundreds of mediators participate in the complicated pathogenesis of sepsis. However, some correlations were found between the prognosis of sepsis and the elevated levels of such proinflammatory cytokines as TNF, IL-6, IL-8 or granulocyte–macrophage colony-stimulating factor (GM-CSF) (Oberholzer et al. 2005; Schefold et al. 2008; Waage et al. 1989).Concentrations of these cytokines increase shortly after LPS stimulation (Ploder et al. 2006). An interesting cytokine that occurs in the circulation in the later phase (12–18 h) of sepsis is a high-mobility group box 1 protein. This ligand is released by the necrotic cells and activated monocytes, as well. Through TLR2 and TLR4 it stimulates other immune cells to produce TNF, activates the endothelium and injures the epithelial cells (Sundén-Cullberg et al. 2005; Wang et al. 1999). There is also some evidence of impaired production of proinflammatory prostaglandins in sepsis (Baenkler et al. 2006). On the other hand, some strong correlations were found between the anti-inflammatory cytokines and the outcome of sepsis. High level of serum IL-10 and the IL-10/TNF ratio indicate high risk of mortality (Schefold et al. 2008). IL-10 is an effective inhibitor of the synthesis of many proinflammatory cytokines and its prolonged massive release may lead to immunoparalysis and secondary infections development (Monneret et al. 2004; Oberholzer et al. 2002). Another important agent in the generation of the immunoparalysis, including monocyte toleration of LPS, is the transforming growth factor β (TGF-β). However, available research did not reveal correlations of TGF-β concentration with clinical data (Oberholzer et al. 2002). Aside from the anti-inflammatory cytokines, many other immunosuppressive mediators were found to be activated in the septic shock, i.e. soluble HLA-G, prostaglandin E2, adrenomedullin, vasoactive intestinal factor, cortisol, norepinephrine and acetylcholine (Gonzalez-Rey et al. 2007; Monneret et al. 2007; Tracey 2007).
Procalcitonin
Procalcitonin (PCT) is a precursor peptide of the hormone calcitonin which is produced in a steady state by C cells of the thyroid and its serum level is very low (Becker et al. 2004). However, it significantly rises during bacteria-caused inflammation, but not in viral or noninfectious inflammation (Harbarth et al. 2001). Many groups have provided evidences for its diagnostic and prognostic utility in sepsis, severe sepsis and septic shock (Fioretto et al. 2008; Meynaar et al. 2011; Tang et al. 2007). PCT is commonly perceived as a marker of a bacterial infection, but it is also a mediator molecule in the sepsis cascade. Although little is known about the biological role of PCT, its synthesis is up-regulated by TNF, a primary sepsis inflammatory mediator (Kettelhack et al. 2000). Peak level of the serum PCT follows the peak of TNF and IL-6 (Dandona et al. 1994). Polymorphonuclear blood cells stimulated by Staphylococcus aureus showed an increased expression of PCT which could be inhibited by the addition of anti-TNF neutralizing antibody (Balog et al. 2002). On the other hand, using a whole blood model, Monneret et al. (2000) observed that the addition of PCT can decrease LPS-induced TNF production. The concentration of PCT, contrary to TNF, remains elevated for much longer after the onset of an infection and it was observed in one study that it peaks a few days before death in non-survivors (Dahaba et al. 2006). It can be hypothesized that the deterioration of the serum PCT before death may reflect the developing immunoparalysis. Monitoring PCT levels is readily available with a commercial immunoluminometric test and for this reason it is a very attractive biomarker, but more studies are needed to prove its utility in monitoring of immunological disturbances. However, the utility of monitoring PCT concentration in sepsis patients has been proved in several randomized clinical trials (Bouadma et al. 2010; Hochreiter et al. 2009; Jensen et al. 2008, 2011; Nobre et al. 2008; Schroeder et al. 2009; Stocker et al. 2010). It allows to optimize and reduce antibiotic use in these patients which contributes to the prevention of selection of drug-resistant pathogens. The decrease of serum PCT concentration indicates the time to cease antibiotic therapy; however, the reduction of time of antibiotic therapy varies in different centers. It should be noted that PCT concentration is an additional parameter and should always be interpreted with the overall clinical status of the patient in mind.
Disruptions in Monocyte Function
It was suggested that the total number of monocytes in peripheral blood of septic patients may be decreased due to common apoptosis of these cells. It may correlate with a favorable prognosis (Giamarellos-Bourboulis et al. 2006). Ex vivo stimulation of monocytes from septic patients by LPS, TLR agonists or whole bacteria results in endotoxin tolerance. This phenomenon was observed in humans ex vivo as a decreased secondary inflammatory response to LPS stimulation after the primary inflammatory response (Granowitz et al. 1993). Moreover, monocytes of septic patients are characterized by a down-regulation of proinflammatory cytokines, like TNF, IL-1, IL-6, IL-12 (Munoz et al. 1991), accompanied by a simultaneous increase in the production of the anti-inflammatory cytokines such as IL-10. All together, those observations suggest that in sepsis, monocytes undergo activation, but the cellular pathways are redirected to the synthesis of anti-inflammatory mediators (Cavaillon and Adib-Conquy 2007).
Another interesting feature of monocytes from septic patients is a decreased surface expression of the HLA-DR molecule, which is a member of the major histocompatibility complex II family (Monneret et al. 2004). Low expression of this molecule indicates the immunosuppressive phase of sepsis. It is the only parameter affected by multiple sepsis mediators, and may be accepted as a highly valuable diagnostic tool. This is reflected by the correlations of HLA-DR expression and plasma concentration of IL-10, IFN-γ, glucocorticosteroids and catecholamines (Le Tulzo et al. 2004; Monneret et al. 2004). Decreased expression of HLA-DR on monocytes predicts severe course of sepsis, occurrence of secondary infections and poor prognosis in critically ill patients (Döcke et al. 2005; Monneret et al. 2006, 2008). In recent years, a growing number of studies have shown the clinical utility of this parameter, which is increasingly popular due to the simplicity of its immunocytochemical staining protocol followed by a flow cytometric analysis (Monneret et al. 2010).
Disruptions in Lymphocytes Functions
T cell lymphocytes play a key role in the anti-infectious defense as both regulators and effectors. It is not surprising that sepsis is related with several profound dysfunctions of these cells. Lymphocytes from septic patients were found to be anergic, i.e. lacking the late-type hyperreactivity in the intradermal tests. This well-known phenomenon is related with the increased vulnerability to secondary infections and mortality (Christou et al. 1995). The depressed functioning of T cells is also revealed in in vitro tests, which demonstrate a low proliferation rate following either specific (anti-CD3 antibody) or non-specific stimulation (phytohemagglutinin) (Lederer et al. 1999). Another feature of lymphocytes in septic immunoparalysis is a series of changes in the expression of surface molecules, which includes up-regulation of proteins responsible for negative signaling in the immune system, such as programmed death 1, CD47 and cytotoxic T lymphocyte antigen 4 (Bandyopadhyay et al. 2007). Accompanying down-regulation of the surface molecule expression, members of stimulatory receptors like CD3 and CD28 is also observed (Hotchkiss and Karl 2003; Venet et al. 2005).
The attention was recently directed to the role of the regulatory CD4+CD25+FoxP3+ T cells (Tregs) in the course of sepsis (Monneret et al. 2003). This specialized subset of helper T cells is capable of modulating the immune response by the following mechanisms: direct induction of apoptosis of cytotoxic lymphocytes (e.g. via Fas/Fas ligand), inhibition of IL-2 and TNF-α release by other cells, and by production of anti-inflammatory cytokines, mainly IL-10 and TGF-β (Jiang and Chess 2004; Venet et al. 2008). Monneret et al. (2003) described that sepsis non-survivors had significantly increased percentage of peripheral blood CD4+CD25+ cells at 7–10 days of the disease. Authors suggested that those T cells are responsible for the development of the immunoparalysis in sepsis (Monneret et al. 2003). Later, the same group proved that the increase of the Tregs percentage is due to the decrease of the other T cells (CD4+CD25−) rather than Tregs proliferation (Venet et al. 2004).
T cells are not the only lymphocytes affected in sepsis, as the count of B cells is also reduced. Interestingly, the number of cytotoxic CD8+ lymphocytes and the natural killer cells was initially not reported to decrease (Hotchkiss et al. 2001). However, some later studies revealed also decrease of the CD8+ cytotoxic T lymphocytes (Gogos et al. 2010; Monserrat et al. 2009). The decreased number of circulating CD8+ T cells was correlated with the survival of sepsis. Loss of lymphocyte subpopulations in the course of sepsis occurs via programmed mechanisms of apoptosis, which can be shown by Annexin V staining (Greineder et al. 2007). According to post-mortem studies, a massive immune cell death takes place in the blood, spleen and lymphoid gut tissue (Hotchkiss et al. 2001).
Changes in Neutrophil Profile
Patients with active infectious process, especially of bacterial etiology, have increased number of immature myeloid lineage cells in their peripheral blood. However, their functional activity is not fully efficient (Taneja et al. 2008). It is worth mentioning their low expression of the chemokine receptors (e.g. CXCR2) and disturbed production of cytokines in vitro (Cummings et al. 1999; Marie et al. 1998). In contrast to other leukocytes, neutrophils are less susceptible to apoptosis leading to their accumulation. Recently, a novel mechanism of neutrophil antibacterial action by formation of neutrophil extracellular traps (NETs) was described (Fuchs et al. 2007). These traps are composed of chromatin and disintegrated granules and it would be of interest to evaluate the NETs formation during sepsis. Mobilization of active neutrophils into the circulation is helpful in eradication of pathogens; however, it contributes to the damage of tissues by the release of enzymes like proteases (Adib-Conquy and Cavaillon 2009).
Disturbances in Phagocytosis
The success of the host defense against bacterial infections is dependent on the effective phagocytosis, which forms a part of the cellular immunity. Phagocytosis is performed mainly by neutrophils and monocytes, but the impairment of phagocytosis during sepsis is actually related to the dysfunctions in neutrophils. The study by Danikas et al. (2008) has elegantly showed that the phagocytic activity of polymorphonuclear cells (PMNs) measured at admission in severe sepsis patients who died is significantly lower than in healthy controls. However, PMNs phagocytic activity in severe sepsis patients who survived is similar to that of the corresponding healthy controls (Danikas et al. 2008). Interestingly, the phagocytic activity of the monocytes measured in the same way in both patients and healthy controls did not differ. Although the role of neutrophils in the pathogenesis of sepsis is not well known, several studies have shown the correlation between the expression of CD64, the high affinity receptor to Fc fragment of IgG, on monocytes and PMNs and the phagocytic activity (Schiff et al. 1997; Wallace et al. 1997). This might prove the close biological relation between those parameters. Analysis of the CD64 expression on monocytes by flow cytometry may be another marker of prognosis and immunological status of sepsis patients.
Coagulation and Immune Disturbances
The relations between coagulation system and the inflammatory response during sepsis are numerous and complicated. As a consequence, activation of platelets, enhancement of plasma procoagulatory activity and attenuation of anticoagulatory activity are all present in septic syndromes. During SIRS endothelium, monocytes and neutrophils are stimulated by TNF and IL-1 to produce the tissue factor (TF). The TF conjugates with coagulation factor VIIa making a TF–FVIIa complex, which activates factor X to the factor Xa. The latter converts prothrombin to thrombin, which in turn converts fibrinogen to fibrin. Thrombin activates other coagulatory factors and the inflammatory cascade as well (Zeerleder et al. 2005). Under normal conditions, thrombin converts the protein C produced in the liver into the activated protein C (APC). This protein binds the endothelium, making a complex with its receptor and thrombomodulin. Together with its cofactor protein S, it inactivates FVa and FIIIa, thus blocking the coagulation cascade. Moreover, APC has anti-inflammatory properties. It inhibits the intracellular NF-κB signal transduction in monocytes, production of inflammatory cytokines, neutrophils recruitment and the mast cells degranulation (Slofstra et al. 2006). Also, APC protects from endothelium “leakage” and migration of the neutrophils (Elphick et al. 2009). Septic patients are deficient of APC and its regulatory mechanisms are disturbed. It has been proved that low level of the serum APC is related with the poorer outcome (Zeng et al. 2004).
Selected Available Modulating Therapies and Potential Interventions in the Immune System
Glucocorticoids
As mentioned at the beginning, the glucocorticoids were thought to be potentially beneficial in the treatment of sepsis because of their immunosuppressive features. When SIRS was thought to be the main reason for the deaths in sepsis, it was tempting to make use of the anti-inflammatory potential of steroids. After conducting several studies, it has been definitely established that the high, immunosuppressive doses of glucocorticoids, e.g. 30 mg of methylprednisolone, do not improve the outcome in sepsis. In fact, it can worsen the prognosis by increasing susceptibility to the secondary infections (Annane et al. 2002). In spite of these results, successive studies with small dose of glucocorticoids in patients requiring catecholamine treatment were conducted. The drug of choice was hydrocortisone at a dose of 200–300 mg/24 h resulting in reduction of 28-day mortality was observed. This outcome was explained by the supplementary role of this treatment in the septic adrenal gland insufficiency (Sprung et al. 2008). However, these encouraging results were unfortunately not confirmed in a recent, big and randomized CORTICUS study. This study enrolled 500 of 800 planned patients in septic shock who required vasopressors for at least 1 h. Patients were randomized to obtain 200 mg/24 h of hydrocortisone for 5 days or placebo for 72 h since the septic shock diagnosis. No survival improvement was confirmed. The only beneficial effect of hydrocortisone treatment was a shortening of the shock phase. According to this study, small doses of glucocorticoids were recommended only in selected cases of septic shock resistant to treatment (Sprung et al. 2008).
Intravenous Immunoglobulins
Most sepsis patients have hypo-gamma-globulinemia mainly due to the low concentration of IgG and these patients were found to have worse survival prognosis (Taccone et al. 2009). Intravenous immunoglobulins (IVIg) act by neutralizing endotoxins, boosting phagocytosis and reducing the cytokine production by mononuclear cells (Negi et al. 2007). Furthermore, new mechanisms of immunomodulation by IVIg are continuously discovered, like the recent effect on the expansion of regulatory T cells (Maddur et al. 2010). Bearing in mind those features, a few studies with IVIg were performed and a little beneficial effect of IVIg on mortality could be observed (Werdan et al. 2007). Unfortunately, most of those trials involved a small group of patients and were not randomized, making them methodologically dubious. It should be noted that in the meta-analysis IgA and IgM IVIg seemed to be more effective (Kreymann et al. 2007). Currently, there is not enough evidence to recommend the wide use of IVIg in the treatment of sepsis in adult patients (Kreymann et al. 2007; Turgeon et al. 2007).
Heparin
Apart from the anticoagulatory effects, heparin acts as an anti-inflammatory agent as well. Not only does it inhibit the release of proinflammatory cytokines, but also stops activation, adhesion and migration of leukocytes. Animal studies have proved its beneficial role in sepsis (Cornet et al. 2007). Therapeutic role of heparin in the clinical setting has been well established in the anticoagulatory prophylaxis. The anti-inflammatory action of heparin has been recently evaluated in a randomized, one-center HETRASE study that enrolled 390 sepsis patients who received 500 U/h of heparin or placebo for 7 days. Although the safety of the unfractionated heparin in sepsis patients was confirmed, the study failed to show the reduction of either 28-day mortality or the length of hospital stay (Jaimes et al. 2009).
Recombinant Human APC
The clinical trials and introduction of the human recombinant APC (rhAPC) into general ICU practice was initially based on the idea of its supplementation due to reduced APC concentration in sepsis. First, the positive effects of rhAPC were assigned mostly to its anticoagulant activities. However, growing body of evidence supports the role of APC as an immunomodulating agent. As we mentioned earlier, APC has an anti-inflammatory potential as it reduces thrombin generation which has strong proinflammatory properties via the protease-activated receptors (PAR), especially PAR-1 and PAR-2 on platelets and endothelium (Ramachandran and Hollenberg 2008). Activation of PAR-1 induces production of chemokines and adhesion molecules (Neyrinck et al. 2009). Another mechanism of an anti-inflammatory action of APC is mediated by the endothelial protein C receptor (EPCR) expressed by leukocytes (monocytes, NK cells, neutrophils) (Joyce et al. 2004) and endothelium (Feistritzer et al. 2006). Ligation of the EPCR on neutrophils and eosinophils decreases chemotactic response and infiltration of tissues (Galley et al. 2008). Also, APC inhibits production of proinflammatory cytokines (TNF, IL-1β, IL-6, IL-8) by LPS-stimulated monocytes (Okajima 2001). APC has additionally anti-apoptotic properties. In the presence of the EPCR, the PAR-1 activation on monocytes inhibits their apoptosis. It was shown that monocytes from rhAPC-treated patients had low Bax/Bcl-2 proteins ratio, which is responsible for the anti-apoptotic state (Bilbault et al. 2007). This effect may support the host response and be beneficial as monocytes in septic patients show high apoptosis rate what contributes to the immunoparalysis, as discussed earlier.
APC have made huge, but not long-lasting career as the first US Food and Drug Administration approved biological drug in sepsis. This protein was the only anti-inflammatory agent that seemed to prove its effectiveness in the treatment of septic shock (Dellinger et al. 2008). A double-blind, randomized, multi-center study evaluated the efficiency of rhAPC in severe sepsis patients. The 96-h-long continuous infusion of rhAPC at a dose of 24 μg/kg showed to be the most effective in decreasing the concentration of D-dimers and IL-6 (Bernard et al. 2001a). Following the study, a third-phase PROWESS trial including 1,690 patients successfully proved the reduction of relative and absolute death risk by 6.1 and 19.4%, respectively (Bernard et al. 2001b). Patients who received rhAPC showed a faster hemodynamic and respiratory improvement with fewer hematological complications. However, patients with low APACHE II score and only one organ dysfunction did not benefit from the treatment (Hodder et al. 2009). Despite these encouraging results, the most recent PROWESS-SHOCK trial, which was a continuation of the PROWESS study, did not confirm the positive therapeutic effects on the morality of sepsis patients. Due to the negative results of the risk–benefit analysis (28-day mortality in rhAPC group was 26.4 vs. 24.2% in the placebo group; Mullard 2011), the company decided to withdraw the rhAPC from market. Whether this potentially immunomodulating agent will receive another chance in immune-status-guided studies remains a question.
GM-CSF and Other Cytokines
In light of the recent research results indicating depression of immune mechanisms, it might be advantageous to apply growth factors in order to restore the host immune functions. In fact, some trials to restore monocyte functions with proteins like IFN-γ, granulocyte colony-stimulating factor (G-CSF), GM-CSF were conducted. Randomized studies on IFN-γ levels in critically ill patients have revealed reduction of both secondary infections and mortality, but only in certain subgroups (Dries et al. 1994). Later study by Döcke et al. (1997) proved positive effects of treatment with IFN-γ on monocyte HLA-DR expression and recovery from infection by septic patients. Studies with GM-CSF in ten patients with acute respiratory distress syndrome (ARDS) have showed improvement of gas exchange parameters, faster ARDS symptoms regression and recovery of alveolar leukocytes (Presneill et al. 2002). Other second-phase GM-CSF study has revealed an increase in leukocyte numbers and HLA-DR expression on monocytes, as well as more effective anti-infectious defense (Rosenbloom et al. 2005). Above studies have not shown an impact of GM-CSF on mortality, but it should be mentioned that immune system status was not evaluated at the time of the growth factor implementation. Similar results were obtained for G-CSF. In vitro and animal studies have encouraged researches to develop clinical studies. Early study on a group of ten patients with ventilator-dependent pneumonia has resulted in mortality rate lower than predicted (Hustinx et al. 1998). Unfortunately, a randomized double-blind, placebo controlled study has not shown any improvements of outcome in septic shock patients (Stephend et al. 2008). A recent meta-analysis of studies with both G-CSF and GM-CSF did not reveal reduction of mortality of septic patients either. However, studies that were analyzed were not coherent and their methodology was not satisfactory (Bo et al. 2011). Stratification of patients by the immune disturbances seems to be crucial to the introduction of specific immunomodulatory therapies.
Statins
Statins are well-known, popular and widely used inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase, the key enzyme of the mevalonate pathway, which in turn inhibits early biosynthesis of cholesterol. In numerous trials statins have shown hypolipidemic activity and reduction of serum C-reactive protein (Arnaud et al. 2005; Ridker et al. 1999). These results resulted in additional research on the impact of statins on immune system modulation. As a consequence, statins were found to be able to inhibit migration and activation of neutrophils and monocytes, as well as capable of reducing the synthesis of protein and cytokines in the acute phase (Cortellare et al. 2002; Krysiak et al. 2003). Furthermore, they improve platelet functions, promote fibrinolysis, induce endothelial NOS expression and increase the number of circulating endothelial progenitors (Laufs et al. 1998; Llevadot et al. 2001). Altogether, these pleiotropic features seem to be a good reason for trials of the application of statins in sepsis. Thus, after interesting outcomes of animal studies (Ando et al. 2000; Yasuda et al. 2006), clinical studies have been performed. Prospective observational study of 11,490 patients with arteriosclerosis subject to either statin therapy or no treatment showed a decreased mortality caused by infections in the statin group (4.1 vs. 0.9%) (Almog et al. 2007). In another study on 361 patients hospitalized due to infection, severe sepsis developed in 19% of non-statin patients, while only 2.4% of those administered statin developed severe sepsis (Almog et al. 2004). Among 2,036 patients admitted with suspicion of infection, the mortality in statin group was 1.9 and 4.4% in the non-statin one (Donnino et al. 2007). Recently, a meta-analysis of patients with sepsis or severe infections also showed reduction of the morality among patients who were receiving statins (Janda et al. 2010). Although the quoted studies are highly encouraging, we are far from the intentional use of statins in the therapy of sepsis. Some problems may arise from the adverse effects of these drugs, including hepatotoxicity and myolysis. Further clinical studies testing safety and efficiency of statins are necessary. At present, the only recommendation can be no interference in statin therapy if the patient received it before developing sepsis (Gao et al. 2008), although this strategy may not bring benefit in survival (Terblanche et al. 2001).
Macrolides
Macrolides are old and well-known group of antibacterial agents with activity against Gram-positive and atypical bacteria. However, observations on patients with chronic inflammatory lung diseases like diffuse panbronchitis and cystic fibrosis suggested additional mechanism of their action which is not related to antimicrobial activity (Schultz 2005). Thus, immunomodulatory mechanisms have been suggested. Indeed macrolides were shown to modulate both innate and adaptive immunity. Patients with community-acquired pneumonia treated with clarithromycin had decreased serum concentration of IL-6, but levels of IL-10 and IFN-γ were elevated (Demartini et al. 2004). In vitro experiments with stimulated monocytes revealed that inhibition of NF-κB pathway is responsible for the changes of cytokines production (Kikuchi et al. 2002). Clarithromycin also affects T lymphocytes leading to a shift towards Th1 response (Williams et al. 2005). In the light of these findings it was tempting to evaluate the effect of macrolides on the course of sepsis. A retrospective-cohort study on 237 patients with sepsis showed that macrolide use was associated with significant reduction of 90-day mortality (Restrepo et al. 2009). Noteworthy, most of the pathogens were not macrolide-susceptible, what supports the immunomodulatory hypothesis. Also, results of the only one available prospective study with clarithromycin in patients with sepsis or ventilator-associated pneumonia are encouraging. Patients who received intravenously clarithromycin presented better resolution of infection and shorter time of weaning from mechanical ventilation (Giamarellos-Bourboulis et al. 2008). These results seem encouraging and require further evaluation in clinical trials and basic studies.
High-Volume Hemofiltration in Sepsis
The most recent trials of therapeutic blocking of a single inflammatory mediator in the developed septic shock have not brought desired effects (Honore et al. 2000). High-volume hemofiltration (HVHF) is a procedure that can be applied in the treatment of septic shock and its use is justified by a growing body of evidence. Early studies on HVHF performed on septic shock patients with acute kidney injury brought significant reduction of mortality among patients who underwent such renal replacement therapy (Cole et al. 2001). This beneficial effect of hemofiltration on the course and outcome of sepsis treatment is related to partial elimination of some shock mediators, such as cytokines (IL-10, IL-6, G-CSF), complement components (C3a, C5a) and other non-protein mediators like leukotrienes and bacterial toxins. Studies have showed the advantage of using the replacement dose of 6 l/h in HVHF over the classic dose of 1 l/h (Ronco et al. 2000). Reduction of plasma concentration of cytokines (C3a, C5a, IL-10), hemodynamic improvement, reduced need for catecholamines and decreased mortality have all been observed. Cole et al. (2001) have showed that performing HVHF with the filtration dose of 35 ml/kg/h resulted in 20% increase in survival. Lately, the strategy of pulse HVHF was tested with promising results (Peng et al. 2010). It is conducted in cycles of 6 h of 80 ml/kg/h of HVHF followed by 18 h of the standard filtration. This preliminary study showed clearance of some sepsis mediators like TNF-α, IL-1, IL-4, IL-6 and IL-10. It has been suggested that the elimination of circulating mediators is an effect of both filtration and absorption to the filter surface (Ronco et al. 2002, 2003). Three theories were proposed to explain the effectiveness of HVHF. According to the first one, the reduction in the highest peak blood concentration of cytokines in the early phase of sepsis can inhibit the inflammatory cascade and the homeostasis disturbance (Honoré et al. 2007). Second, the concept of the immunomodulatory threshold assumes that removal of blood cytokines leads to the shift of tissue-concentrated cytokines into the circulation (Di Carlo and Alexander 2005). This may explain why not all studies have been able to show changes in the circulating levels of cytokines, although obtaining clinical improvement. The last hypothesis is based on the observation that HVHF can increase the lymphatic plasma flow even 40-fold, causing removal of large amount of cytokines from tissues (Di Carlo and Alexander 2005). Despite promising results, insufficient evidence is available concerning adequate strategies, doses and best time to start HVHF to recommend it in septic patients without septic acute kidney injury. However, HVHF at a dose of 35 ml/kg/h should be applied in septic patient requiring renal replacement therapies (Honoré et al. 2009).
Conclusion
Currently, the new strategies reviewed above (except rhAPC for a few years) are not systematically used in sepsis treatment on a large scale. The emphasis is routinely put on the early diagnosis of sepsis, especially outside ICU, as it enables implementation of the recommended treatment, eventually reducing morality. Releasing and introducing international guidelines for the diagnosis and treatment of severe sepsis in 2004 has led to marked improvements in the outcomes and overall lower mortality. After 4 years the guidelines were updated, but no new drugs or procedures have been introduced. Changes concern more precise distinction between “recommendations” and “suggestions” based on the “risk–benefit analysis”.
Based on the previously released “ventilatory bundles” and their beneficial impact on the course of mechanically ventilated patients, septic bundles were introduced. “Sepsis bundle” is a set of treatment procedures, which implemented together are more effective than in separation. The choice of the elements of the bundles is based on strong evidence and the implementation of the bundles should be generally accepted. In the treatment of sepsis, two bundles have been set: the Sepsis Resuscitation Bundle and the Sepsis Management Bundle (Townsend et al. 2008) (Table 2).
Although the immune reaction in sepsis is generalized and clinically similar in most patients, some papers bring evidence that sepsis immunopathology differs between patients. It was observed that infections by Gram-positive pathogenes lead to higher decrease in CD4+, CD8+, NK cells and total T lymphocytes than Gram-negative sepsis. Also, the pattern of immune recovery in Gram-positive sepsis was different and slower than in Gram-negative infections (Holub et al. 2003). On the other hand, a recent study on 505 septic patients showed differences in the circulating immune cells depending on the type of the primary infection (Gogos et al. 2010). Main differences were found by comparing sepsis severity in these infections, i.e. septic shock due to pneumonia was characterized by lower counts of NK cells, CD4+, CD8+ and B cells than sepsis due to intraabdominal infections. Septic shock due to intraabdominal infection caused lower counts of HLA-DR+ monocytes, CD8+ and CD4+ lymphocytes than sepsis of the same origin.
This heterogeneity of the immune system response during sepsis provides an additional argument for careful monitoring of the immune response during therapy. It can also provide a possible explanation why the implementation of the resuscitation bundles, but not the therapeutic bundles is an independent predictor of survival (Castellanos-Ortega et al. 2010). The resuscitation bundles are beneficial in each type of sepsis, as they are aimed at maintaining perfusion and early antibacterial therapy, while the therapeutic bundles involve the use of immunomodulating agents, glucocorticosteroids and APC which might be helpful only in selected patients.
The implementation of the new immunomodulatory therapies should also be supported by strong-evidence results. However, in special cases, some of the reviewed treatment possibilities can be used as the salvage therapy. Clearly, continuous efforts should be made in the field of sepsis pathophysiology and the development of new therapeutic strategies.
In this review we have analyzed the studies of human sepsis. Although many interesting results have been obtained from animal models of sepsis, there are many differences between animal and human sepsis and the transition of some therapeutic regimens from murine models to clinical setting have failed. Therefore, we chose to focus on the results of the research on human sepsis and clinical studies.
Abbreviations
- APC:
-
Activated protein C
- ARDS:
-
Acute respiratory distress syndrome
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- TF:
-
Tissue factor
- HVHF:
-
High-volume hemofiltration
- HLA:
-
Human leukocyte antigen
- ICU:
-
Intensive care units
- IFN-γ:
-
Interferon gamma
- IVIg:
-
Intravenous immunoglobulins
- LPS:
-
Lipopolysaccharide
- NETs:
-
Neutrophil extracellular traps
- PAMPs:
-
Pathogen-associated molecular patterns
- PARRs:
-
Pattern recognition receptors
- PCT:
-
Procalcitonin
- PMNs:
-
Polymorphonuclear cells
- SIRS:
-
Systemic inflammatory response syndrome
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
References
Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101:36–47
Almog YM, Shefer AM, Novack VM et al (2004) Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 110:880–885
Almog Y, Novack V, Eisinger M et al (2007) The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med 35:372–378
Ando H, Takamura T, Ota T et al (2000) Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther 294:1043–1046
Angus DC, Linde-Zwirble WT (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Annane D, Sébille V, Charpentier C et al (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871 (erratum in JAMA 2008; 300:1652)
Arnaud C, Burger F, Steffens S et al (2005) Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct anti-inflammatory effects of statins. Arterioscler Thromb Vasc Biol 25:1231–1236
Baenkler M, Leykauf M, Johj S (2006) Functional analysis of eicosanoids from white blood cells in sepsis and SIRS. J Physiol Pharmacol 57(Suppl 12):25–33
Balog A, Ocsovszki I, Mándi Y (2002) Flow cytometric analysis of procalcitonin expression in human monocytes and granulocytes. Immunol Lett 84:199–203
Bandyopadhyay G, De A, Laudanski K et al (2007) Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med 35:794–801
Becker KL, Nylén ES, White JC et al (2004) Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 89:1512–1525
Bernard GR, Ely EW, Wright TJ et al (2001a) Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med 29:2051–2059
Bernard GR, Vincent JL, Laterre PF et al (2001b) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Bilbault P, Lavaux T, Launoy A et al (2007) Influence of drotrecogin alpha (activated) infusion on the variation of Bax/Bcl-2 and Bax/Bcl-xl ratios in circulating mononuclear cells: a cohort study in septic shock patients. Crit Care Med 35:69–75
Bo L, Wang F, Zhu J et al (2011) Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit Care 15:R58
Bone RC (1996) Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 24:1125–1128
Bone RC, Balk RA, Cerra FB et al (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Bouadma L, Luyt CE, Tubach F et al (2010) Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375:463–474
Castellanos-Ortega A, Suberviola B, García-Astudillo LA et al (2010) Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 38:1036–1043
Cavaillon JM, Adib-Conquy M (2007) Determining the degree of immunodysregulation in sepsis. Contrib Nephrol 156:101–111
Christou NV, Meakins JL, Gordon J et al (1995) The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg 222:534–546
Cole L, Bellomo R, Journois D et al (2001) High-volume haemofiltration in human septic shock. Intensive Care Med 27:978–986
Cornet AD, Smit EG, Beishuizen A et al (2007) The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost 98:579–586
Cortellare M, Cofrancesco E, Arbustini E et al (2002) Atorvastatin and thrombogeneicity of the carotid atherosclerotic plaque: the ATROCAP study. Throm Haemost 88:41–47
Cummings CJ, Martin TR, Frevert CW et al (1999) Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol 162:2341–2346
Dahaba AA, Hagara B, Fall A et al (2006) Procalcitonin for early prediction of survival outcome in postoperative critically ill patients with severe sepsis. Br J Anaesth 97:503–508
Dandona P, Nix D, Wilson MF et al (1994) Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 79:1605–1608
Danikas D, Karakantza M, Theodorou G et al (2008) Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 154:87–97
Dellinger RP, Levy MM, Carlet JM et al (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Demartini G, Esposti D, Marthyn P et al (2004) Effect of multiple doses of clarithromycin and amoxicillin on IL-6, IFNgamma and IL-10 plasma levels in patients with community acquired pneumonia. J Chemother 16:82–85
Di Carlo JV, Alexander SR (2005) Hemofiltration for cytokine-driven illness: the mediator delivery hypothesis. Int J Artif Organs 28:777–786
Döcke WD, Randow F, Syrbe U et al (1997) Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 6:678–681
Döcke WD, Höflich C, Davis KA et al (2005) Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem 51:2341–2347
Donnino MW, Cocchi MN, Howell MN et al (2007) Statin therapy in patients with sepsis. Acad Emerg Med 16:230–234
Draisma A, Dorresteijn M, Pickkers P et al (2008) The effect of systemic iNOS inhibition during human endotoxemia on the development of tolerance to different TLR-stimuli. Innate Immun 14:153–159
Dries DJ, Jurkovich GJ, Maier RV et al (1994) Effect of interferon gamma on infection-related death in patients with severe injuries. A randomized, double-blind, placebo-controlled trial. Arch Surg 129:1031–1041
Elphick GF, Sarangi PP, Hyun YM et al (2009) Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood 113:4078–4085
Feistritzer C, Schuepbach RA, Mosnier LO et al (2006) Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem 281:20077–20084
Fioretto JR, Martin JG, Kurokawa CS et al (2008) Interleukin-6 and procalcitonin in children with sepsis and septic shock. Cytokine 43:160–164
Fuchs TA, Abed U, Goosmann C et al (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241
Galley HF, El Sakka NE, Webster NR et al (2008) Activated protein C inhibits chemotaxis and interleukin-6 release by human neutrophils without affecting other neutrophil functions. Br J Anaesth 100:815–819
Gao F, Linhartowa L, Jahnston AM et al (2008) Statins and sepsis. Br J Anaesth 100:288–298
Giamarellos-Bourboulis EJ, Routsi C, Plachouras D et al (2006) Early apoptosis of blood monocytes in the septic host: is it a mechanism of protection in the event of septic shock? Crit Care 10:R76
Giamarellos-Bourboulis EJ, Pechère JC, Routsi C et al (2008) Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin Infect Dis 46:1157–1164
Gogos C, Kotsaki A, Pelekanou A et al (2010) Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care 14:R96
Gonzalez-Rey E, Chorny A, Delgado M (2007) Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol 7:52–63
Granowitz EV, Porat R, Mier JW et al (1993) Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol 151:1637–1645
Greineder CF, Nelson PW, Dressel AL et al (2007) In vitro and in silico analysis of annexin V binding to lymphocytes as a biomarker in emergency department sepsis studies. Acad Emerg Med 14:763–771
Harbarth S, Holeckova K, Froidevaux C, Geneva Sepsis Network et al (2001) Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med 164:396–402
Hochreiter M, Köhler T, Schweiger AM et al (2009) Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 13:R83
Hodder RV, Hall R, Russell JA et al (2009) Early drotrecogin alpha (activated) administration in severe sepsis is associated with lower mortality: a retrospective analysis of the Canadian ENHANCE cohort. Crit Care 13:R78
Holub M, Klucková Z, Helcl M et al (2003) Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clin Microbiol Infect 9:202–211
Honore PM, Jamez J, Wauthier M et al (2000) Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med 28:3581–3587
Honoré PM, Joannes-Boyau O, Gressens B (2007) Blood and plasma treatments: the rationale of high-volume hemofiltration. Contrib Nephrol 156:387–395
Honoré PM, Joannes-Boyau O, Collin V et al (2009) Continuous hemofiltration in 2009: what is new for clinicians regarding pathophysiology, preferred technique and recommended dose? Blood Purif 28:135–143
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Eng J Med 348:138–150
Hotchkiss RS, Tinsley KW, Swanson PE et al (2001) Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 166:6952–6963
Hustinx WN, van Kessel CP, Heezius E et al (1998) Effects of granulocyte colony-stimulating factor (G-CSF) treatment on granulocyte function and receptor expression in patients with ventilator-dependent pneumonia. Clin Exp Immunol 112:334–340
Jaimes F, De La Rosa G, Morales C et al (2009) Unfractioned heparin for treatment of sepsis: a randomized clinical trial (The HETRASE Study). Crit Care Med 37:1185–1196
Janda S, Young A, Fitzgerald JM et al (2010) The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care 25:656.e7–e22
Jensen JU, Lundgren B, Hein L et al (2008) The Procalcitonin and Survival Study (PASS)—a randomised multi-center investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and pro-active diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. Calculated sample size (target population): 1000 patients. BMC Infect Dis 8:91
Jensen JU, Hein L, Lundgren B et al (2011) Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 39:2048–2058
Jiang H, Chess L (2004) An integrated model of immunoregulation mediated by regulatory T cell subsets. Adv Immunol 83:253–288
Joyce DE, Nelson DR, Grinnell BW (2004) Leukocyte and endothelial cell interactions in sepsis: relevance of the protein C pathway. Crit Care Med 32(5 Suppl):S280–S286
Kettelhack C, Hohenberger P, Schulze G et al (2000) Induction of systemic serum procalcitonin and cardiocirculatory reactions after isolated limb perfusion with recombinant human tumor necrosis factor-alpha and melphalan. Crit Care Med 28:1040–1046
Kikuchi T, Hagiwara K, Honda Y et al (2002) Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J Antimicrob Chemother 49:745–755
Kreymann KG, de Heer G, Nierhaus A et al (2007) Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 35:2677–2685
Krysiak R, Okopien B, Herman ZS (2003) Effects of HMG-CaA reductase inhibitors on coagulation and fibrinolysis processes. Drugs 63:1821–1854
Laufs U, La Fata V, Plutzky J et al (1998) Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97:1129–11235
Le Tulzo Y, Pangault C, Amiot L et al (2004) Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med 169:1144–1151
Lederer JA, Rodrick ML, Mannick JA (1999) The effects of injury on the adaptive immune response. Shock 11:153–159
Levy MM, Dellinger PR, Townsend SR et al (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36:222–231
Llevadot J, Murasawa S, Kureishi Y et al (2001) HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest 108:399–405
Maddur MS, Othy S, Hegde P et al (2010) Immunomodulation by intravenous immunoglobulin: role of regulatory T cells. J Clin Immunol 30(Suppl 1):S4–S8
Marie C, Muret J, Fitting C et al (1998) Reduced ex vivo interleukin-8 production by neutrophils in septic and nonseptic systemic inflammatory response syndrome. Blood 91:3439–3446
Meynaar IA, Droog W, Batstra M et al (2011) In critically III patients, serum procalcitonin is more useful in differentiating between sepsis and SIRS than CRP, Il-6, or LBP. Crit Care Res Pract 2011:594645
Monneret G, Pachot A, Laroche B et al (2000) Procalcitonin and calcitonin gene-related peptide decrease LPS-induced TNF production by human circulating blood cells. Cytokine 12:762–764
Monneret G, Debard AL, Venet F et al (2003) Marked elevation of human circulating CD4+ CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med 31:2068–2071
Monneret G, Finck ME, Venet F et al (2004) The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95:193–198
Monneret G, Lepape A, Voirin N et al (2006) Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32:1175–1178
Monneret G, Voirin N, Krawice-Radanne I et al (2007) Soluble human leukocyte antigen-G5 in septic shock: marked and persisting elevation as a predictor of survival. Crit Care Med 35:1942–1947
Monneret G, Venet F, Pachot A et al (2008) Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 14:64–78
Monneret G, Venet F, Meisel C et al (2010) Assessment of monocytic HLA-DR expression in ICU patients: analytical issues for multicentric flow cytometry studies. Crit Care 14:432
Monserrat J, de Pablo R, Reyes E et al (2009) Clinical relevance of the severe abnormalities of the T cell compartment in septic shock patients. Crit Care 13:R26
Mullard A (2011) Drug withdrawal sends critical care specialists back to basics. Lancet 378:1769
Munoz C, Carlet J, Fitting C et al (1991) Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest 88:1747–1754
Negi VS, Elluru S, Sibéril S et al (2007) Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol 27:233–245
Neyrinck AP, Liu KD, Howard JP et al (2009) Protective mechanisms of activated protein C in severe inflammatory disorders. Br J Pharmacol 158:1034–1047
Nobre V, Harbarth S, Graf JD et al (2008) Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 177:498–505
Oberholzer A, Oberholzer C, Moldawer LL (2002) Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med 30(1 Suppl):S58–S63
Oberholzer A, Souza SM, Tschoeke SK et al (2005) Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock 23:488–493
Okajima K (2001) Regulation of inflammatory responses by natural anticoagulants. Immunol Rev 184:258–274
Opal SM, Fisher CJ, Dhainaut JF et al (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind placebo-controlled, multicenter trial. Crit Care Med 25:1115–1124
Peng Z, Pai P, Han-Min W et al (2010) Evaluation of the effects of pulse high-volume hemofiltration in patients with severe sepsis: a preliminary study. Int J Artif Organs 33:505–511
Ploder M, Pelinka L, Schmuckenschlager C et al (2006) Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock 25:129–134
Presneill JJ, Harris T, Stewart AG et al (2002) A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med 166:138–143
Ramachandran R, Hollenberg MD (2008) Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol 153(Suppl 1):S263–S282
Reinhart K, Menges T, Gardlund B et al (2001) Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES Study. Crit Care Med 29:765–769
Restrepo MI, Mortensen EM, Waterer GW et al (2009) Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J 33:153–159
Ridker PM, Rifai N, Pfeffer MA et al (1999) Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 100:230–235
Ronco C, Bellomo R, Homel P et al (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
Ronco C, Ricci Z, Bellomo R (2002) Importance of increased ultrafiltration volume and impact on mortality: sepsis and cytokine story and the role for CVVH. EDTNA ERCA J Suppl 2:13–18
Ronco C, Tetta C, Mariano F et al (2003) Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs 27:792–801
Rosenbloom AJ, Linden PK, Dorrance A et al (2005) Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest 127:2139–2150
Schefold JC, Hasper D, von Haehling S et al (2008) Interleukin-6 serum level assessment using a new qualitative point-of-care test in sepsis: a comparison with ELISA measurements. Clin Biochem 41:893–898
Schiff DE, Rae J, Martin TR et al (1997) Increases phagocyte CD64 expression and improved Fc-receptor mediated phagocytosis following in vivo recombinant human interferon-γ treatment of normal healthy subjects. Blood 90:3187–3194
Schroeder S, Hochreiter M, Koehler T et al (2009) Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 394:221–226
Schultz MJ (2005) Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchitis and cystic fibrosis. J Antimicrob Chemother 54:21–28
Slofstra SH, ten Cate H, Spek CA (2006) Signal transduction induced by activated protein C: no role in protection against sepsis? Trends Mol Med 12:374–381
Sprung CL, Annane D, Keh D, CORTICUS Study Group et al (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124
Stephend DP, Thomas JH, Higgins A et al (2008) Randomized, double-blind, placebo-controlled trial of granulocyte colony-stimulating factor on patients with septic shock. Crit Care Med 36:448–454
Stocker M, Hop WC, van Rossum AM (2010) Effect of procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: a multi-centre randomized superiority and non-inferiority intervention study. BMC Pediatr 10:89
Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A et al (2005) Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med 33:564–573
Taccone FS, Stordeur P, De Backer D et al (2009) Gamma-globulin levels in patients with community-acquired septic shock. Shock 32:379–385
Taneja R, Sharma AP, Hallett MB et al (2008) Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock 30:618–622
Tang BM, Eslick GD, Craig JC et al (2007) Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 7:210–217
Terblanche MJ, Pinto R, Whiteley C et al (2001) Statins do not prevent acute organ failure in ventilated ICU patients: single-centre retrospective cohort study. Crit Care 15:R74
Torgersen C, Moser P, Luckner G et al (2009) Macroscopic postmortem findings in 235 surgical intensive care patients. Anesth Analg 108:1841–1847
Townsend SR, Schorr C, Levy MM (2008) Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med 29:721–733
Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117:289–296
Turgeon AF, Hutton B, Fergusson DA et al (2007) Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 146:193–203
Venet F, Pachot A, Debard AL et al (2004) Increased percentage of CD4+ CD25+ regulatory T cells during septic shock is due to the decrease of CD4+ CD25− lymphocytes. Crit Care Med 32:2329–2331
Venet F, Bohé J, Debard AL et al (2005) Both percentage of gammadelta T lymphocytes and CD3 expression are reduced during septic shock. Crit Care Med 33:2836–2840
Venet F, Chung CS, Monneret G et al (2008) Regulatory T cell populations in sepsis and trauma. J Leukoc Biol 83:523–535
Waage A, Brandtzaeg P, Halstensen A et al (1989) The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med 169:333–338
Wallace PK, Keler T, Coleman K et al (1997) Humanized mab H22 binds the human high affinity Fc receptor for IgG(FcgammaR1), blocks phagocytosis and modulates receptor expression. J Leukoc Biol 62:469–479
Wang H, Bloom O, Zhang M et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Werdan K, Pilz G, Bujdoso O, Score-Based Immunoglobulin Therapy of Sepsis (SBITS) Study Group et al (2007) Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 35:2693–2701
Williams AC, Galley HF, Watt AM et al (2005) Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother 56:502–506
Yasuda H, Yuen PS, Hu X et al (2006) Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69:1535–1542
Zeerleder S, Hack CE, Wuillemin WA (2005) Disseminated intravascular coagulation in sepsis. Chest 128:2864–2875
Zeng W, Matter WF, Yan SB et al (2004) Effect of drotrecogin alfa (activated) on human endothelial cell permeability and Rho kinase signaling. Crit Care Med 32(5 Suppl):S302–S308
Acknowledgments
This paper was supported by Grant No. N N401 039037 from the Polish Ministry of Science and Higher Education (G. Hoser) and by European Union Structural Funds, “Innovative methods of stem cells applications in medicine”, Innovative Economy Operational Programme, grant no. POIG 01.02-00-109/09. The authors would like to thank Chris Kozak from Department of Zoology, University of Cambridge (UK), for his invaluable help in correction of the manuscript and excellent comments.
Conflict of interest
The authors declare no conflicts of interests in relation to this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Skirecki, T., Borkowska-Zielińska, U., Złotorowicz, M. et al. Sepsis Immunopathology: Perspectives of Monitoring and Modulation of the Immune Disturbances. Arch. Immunol. Ther. Exp. 60, 123–135 (2012). https://doi.org/10.1007/s00005-012-0166-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-012-0166-1