Abstract
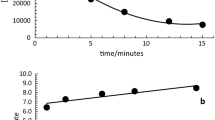
Sorption data were obtained with a Matawan soil and the following chromium (III) organic complexes: chromium (III) ascorbate, chromium (III) glutamate, chromium (III) histidine, chromium (III) mandelate, chromium (III) citrate, chromium (III) cysteine, chromium (III) serine, chromium (III) pyruvate and chromium (III) oxalate. The influence of pH (2–12), ionic strength (0.005–1 M) and concentration of sorbate (1–10 mg/L) on the extent of sorption was evaluated. The pH value did not influence the percent sorption at environmentally relevant pH 7. Ionic strength between 0.005 and 0.01 M KNO3 did not influence the sorption. Sorption and desorption data obtained at pH 7, 0.01 M KNO3 and 1–10 mg/L for each chromium (III) organic complex were analyzed using Freundlich and Langmuir models. The Freundlich model provided good fits for all of the chromium (III) organic complexes. Sorption data for chromium (III) glutamate, chromium (III) pyruvate, chromium (III) oxalate, chromium (III) cysteine, chromium (III) ascorbate and chromium (III) citrate were described well by the Langmuir model. Estimates for the saturated sorption capacities were 141, 70.9, 36.5, 35.5, 28.6 and 4.4 μg/g, respectively. It was not possible to desorb significant amounts of the previously sorbed chromium (III) organic complexes. At the same pH, ionic strength and solid:liquid ratio, the order of the observed sorption to the Matawan soil from highest to lowest was chromium (III) mandelate, chromium (III) glutamate, chromium (III) histidine, chromium (III) cysteine, chromium (III) serine, chromium (III) pyruvate, chromium (III) oxalate, chromium (III) ascorbate and chromium (III) citrate.
Similar content being viewed by others
References
Adams, T. K.; Saydam, N.; Steiner, F.; Schaffner, W.; Freedman, J. H., (2002). Activation of gene expression by metal-responsive signal transduction pathways. Environ. Health Perspect., 110 (5), 813–817 (5 pages).
Ahern, F.; Eckert, J. M.; Payne, N. C.; Williams, K. C, (1985). Speciation of chromium in seawater. Anal. Chim. Acta., 175, 147–151 (5 pages).
Buerge-Weirich, D.; Behra, P.; Sigg, L., (2003). Adsorption of copper, nickel and cadmium on goethite in the presence of organic ligands. Aquat. Geochem., 9 (2), 65–85 (21 pages).
Carbonaro, R.; Stone, A. T., (2005). Speciation of chromium (III) and cobalt (III) (amino) carboxylate complexes using capillary electrophoresis. Anal. Chem., 77 (1), 155–164 (10 pages).
Clescerl, L. S.; Greenberg, A. E.; Eaton, A. D., (1989). Standard methods for examination of water and wastewater, 17th. Ed., American Public Health Association.
Costa, M., (2003). Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharma., 188 (1), 1–5 (6 pages).
Davis, A.; Kempton, J. H.; Nicholson, A.; Yare, B., (1994). Groundwater transport of arsenic and chromium at a historical tannery. Woburn, Massachusetts, U.S.A. Appl. Geochem., 9 (5), 569–582 (14 pages).
Freundlich, H., (1926). Colloid and capillary chemistry, Methuen. London, 397-414.
Fukushima, M.; Nakayasu, K.; Tanaka, S.; Nakamura, H. (1995). Chromium (III) binding abilities of humic acids. Anal. Chim. Acta., 317 (1–3), 195-206 (12 pages).
Gaspar, A.; Buglyo, P., (2000). Separation and kinetic study of chromium (III) chlorocomplexes by capillary electrophoresis. Chromatographia, 51 (1), 143–147 (5 pages).
Ge, Y.; Hendershot, W., (2004). Evaluation of soil surface charge using the back-titration technique. Soil Sci. Soc. Am. J., 68, 82–88 (7 pages).
Holdway, D. A., (1988) The toxicity of Cr to fish, in chromium in the natural and human environments. Nriagu, J. O.; Nieboer, E., Eds., John Wiley and Sons, Advances in Environmental Science and Technology No. 20; Wiley-Interscience: New York, 369–397.
Howe, J. A.; Loeppe R. H.; DeRose, V. J.; Hunter, D. B.; Bertsch, P. M., (2003). Localization and speciation of chromium in subterranean clover using XRF, XANES and EPR Spectroscopy. Environ. Sci. Tech., 37 (18), 4091–4097 (7 pages).
Huang, C.; Zhang, Q.; Li, J.; Shi, X.; Castranova, V.; Ju, G.; Costa, M.; Dong, Z., (2001). Involvement of Erks activation in cadmium-induced AP-1 transactivation in vitro and in vivo. Mol. Cell. Biochem., 222(1–2), 141–147 (7 pages).
Icopini, G A.; Long, D. T., (2002). Speciation of aqueous chromium by use of solid-phase extractions in the field. Environ. Sci. Tech., 36 (13), 2994–2999 (5 pages).
Kaczynski, S. E.; Kieber, R. J., (1994). Hydrophobie C18 bound organic complexes of chromium and their potential impact on the geochemistry of chromium in natural waters. Environ. Sci. Tech., 28 (5), 799–804 (5 pages).
Langmuir, I., (1918). The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc, 40, 1361–1403 (42 pages).
Mattuck, R.; Nikolaidis, N. P., (1996). Chromium mobility in freshwater wetlands. J. Comtam. Hydrol., 23 (3), 213–232 (20 pages).
Nakayama, E.; Kuwamoto, T.; Tokoro, H.; Fujinaga, T., (1981). Dissolved state of chromium in seawater. Nature, 290, 768–770 (3 pages).
Nieboer, E.; Jusys, A. A., (1988). Biological chemistry of Cr, in chromium in the natural and human environments. Nriagu, J. O.; Nieboer, E., Eds., John Wiley and Sons, Advances in Environmental Science and Technology No. 20; Wiley-Interscience: New York, 21–79.
Ogundiran, O. O.; Afolabi, T. A., (2008). Assessment of the physicochemical parameters and heavy metals toxicity of leachates from municipal solid waste open dumpsite. Int. J. Environ. Sci. Tech., 5 (2), 243–250.
Oshida, P. S.; Word, L. S.; Mearns, A. J., (1981). Effects of hexavalent and trivalent Cr on the reproduction of Neanthes arenaceodentata (polychaeta). Mar. Environ. Res., 5, 41–49 (9 pages).
Pearson, R. G, (1963). Hard and soft acids and bases. J. Am. Chem. Soc, 85, 3533–3539 (6 pages).
Petruzzelli, G; Guidi, G; Lubrano, L., (1985). Ionic strength effect on heavy metal adsorption by soil. Commun. Soil Sci. Plan., 16 (9), 971–986 (16 pages).
Puzon, G J.; Roberts, A. G; Kramer, D. M.; Xun, L., (2005). Formation of soluble organo-Chromium (III) complexes after chromate reduction in the presence of cellular organics. Environ. Sci. Tech., 39 (8), 2811–2817 (7 pages).
Puzon, G J.; Tokala, R. K.; Zhang, H.; Yonge, D.; Peyton, B. M; Xun, L., (2008). Mobility and recalcitrance of organo—chromium (III) complexes. Chemosphere, 70 (11), 2054–2059 (5 pages).
Richard, F. C.; Bourg, A. C. M., (1991). Aqueous geochemistry of chromium: A review. Water Res., 25 (7), 807–816 (10 pages).
Sass, B. M.; Rai, D., (1987). Solubility of amorphous chromium Ill-iron (III) hydroxide solid solutions. Inorg. Chem., 26 (14), 2228–2232 (5 pages).
Sivakumar, S.; Subbhuraam, C. V., (2005). Toxicity of chromium (III) and chromium (VI) to the earthworm Eisenia fetida. Ecotox. Environ. Safe., 62 (1), 93–98 (6 pages).
Shah, B. A.; Shah, A. V.; Singh, R. R., (2009). Sorption isotherms and kinetics of chromium uptake from wastewater using natural sorbent material. Int. J. Environ. Sci. Tech., 6 (1), 77–90 (14 pages).
Shrestha, R.; Fischer, R.; Sillanpaa, M., (2007). Investigations on different positions of electrodes and their effects on the distribution of Cr at the water sediment interface. Int. J. Environ. Sci. Tech., 4 (4), 413–420 (7 pages).
Stumm, W.; Morgan, J. J., (1996). Aquatic chemical equilibrium and rates in natural water. 3rd. Ed., John Wiley and Sons, Inc., New York, Wiley-Interscience, 1022.
Vitale, R. J.; Mussoline, G R.; Rinehimer, K. A., (1997). Environmental monitoring of chromium in air, soil and water. Regul. Toxicol. Pharm., 26 (1–2), 80–85 (6 pages).
von Gunten, H. R.; Kull, T. P., (1986). Infiltration of inorganic compounds from the glatt river. Switzerland, into a groundwater aquifer. Water Air Soil Pollut., 29 (3), 333–346 (14 pages).
Walsh, A. R.; O’Halloran, J., (1996). Chromium speciation in tannery effluent-I, an assessment of techniques and the role of organic chromium (III) complexes. Water Res., 30 (10), 2393–2400 (8 pages).
Walsh, A. R.; O’Halloran, J., (1997). The accumulation of chromium by mussels Mytilus edulis (L.) as a function of valency, solubility and ligation. Mar. Environ. Res., 43 (1–2), 41–53 (13 pages).
Wang, W. X.; Griscom, S. B.; Fisher, N. S., (1997). Bioavailability of Cr (III) and Cr (VI) to marine mussels from solute and particulate pathways. Environ. Sci. Tech., 31 (2), 603–611 (9 pages).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Z., Wadhawan, A. & Bouwer, E.J. Sorption behavior of nine chromium (III) organic complexes in soil. Int. J. Environ. Sci. Technol. 7, 1–10 (2010). https://doi.org/10.1007/BF03326111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03326111