Abstract
-
▲ Salmeterol/fluticasone propionate is a fixed-dose combination of the long-acting β2-adrenoceptor agonist salmeterol and the corticosteroid fluticasone propionate and is inhaled via the Diskus™1 powder inhaler.
-
▲ In three randomized, double-blind, 24-week or 52-week studies in >2850 patients with chronic obstructive pulmonary disease (COPD), administration of salmeterol/fluticasone propionate 50/250μg twice daily (in one study) and salmeterol/fluticasone propionate 50/500μg twice daily (in the other studies) provided greater improvement in lung function than placebo or either component alone at the same nominal dosage.
-
▲ Both strengths of the combination product administered twice daily resulted in clinically meaningful increases in scores in health-related quality-of-life questionnaires that were specific for respiratory disease. Improvements in this and almost all other secondary measures of efficacy, including symptomatic outcomes, were significantly greater with the combination product than with placebo.
-
▲ Administration of salmeterol/fluticasone propionate as a combination product did not result in any untoward interactions that affected the pharmacodynamic, pharmacokinetic or tolerability profiles of the individual components.
-
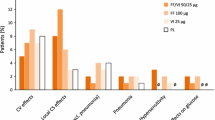
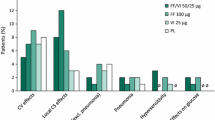
▲ Candidiasis, hoarseness/dysphonia, throat irritation and headache occurred more frequently with salmeterol/fluticasone propionate than with placebo in patients with COPD.



Similar content being viewed by others
References
American Lung Association. Epidemiology and Statistics Unit. Trends in chronic bronchitis and emphysema: morbidity and mortality [online]. Available from URL: http://www.lungusa.org/data/copd/copdl.pdf [Accessed 2002 Apr 9]
Celli BR, Snider GL, Heffner J, et al., on behalf of the American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995 Nov; 152 (5 Suppl. 2): S77–S120
Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000; 343(4): 269–80
Siafakas NM, Vermeire P, Pride NB, et al., on behalf of the European Respiratory Society Task Force. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995; 8: 1398–420
Calhoun J, Coetzer H. Respiratory disease management: yesterday’s failures, tomorrow’s successes. Dis Manage Health Outcomes 2001; 9Suppl. 1: 21–7
Kawane H. Smoking as a risk factor for COPD and prognosis after smoking cessation [in Japanese]. Nippon Rinsho 1999 Sep; 57(9): 1959–64
Pride N. Smoking cessation: effects on symptoms, spirometry and future trends in COPD. Thorax 2001; 56Suppl. II: ii7–ii10
Stuart-Harris C, Bishop JM, Clark TJH, et al., on behalf of the Medical Research Council Working Party. Long term domicilary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981 Mar 28; 8222: 681–6
Crockett AJ, Cranston JM, Moss JR, et al. Survival on long-term oxygen therapy in chronic airflow limitation: from evidence to outcomes in the routine clinical setting. Intern Med J 2001; 31: 448–54
Pauwels RA, Buist AS, Calverley PMA, et al., on behalf of the GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD): Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–76
Burge S. Should inhaled corticosteroids be used in the long term treatment of chronic obstructive pulmonary disease? Drugs 2001; 61(11): 1535–44
Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 580–4
Burge PS, Calverley PMA, Jones PW, et al., on behalf of the ISOLDE Study Investigators. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000 May 13; 320: 1297–303
Soriano JB, Vestbo J, Pride NB, et al. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J. In press
Jarvis B, Markham A. Inhaled salmeterol: a review of its efficacy in chronic obstructive pulmonary disease. Drugs Aging 2001; 18(6): 441–72
Watkins M, Wire P, Yates J, et al. Sustained FEV1 increases in COPD patients induced by salmeterol 50mcg twice daily via the Diskus inhaler [abstract]. Am J Resp Crit Care Med 2002 Apr; 165(8 Suppl.): A228
Knobil K, Kalberg C, Emmett A, et al. The bronchodilator response to salmeterol is maintained with regular, long-term use in patients with COPD [abstract]. Am J Resp Crit Care Med 2002 Apr; 165(8 Suppl.): A229
Holliday SM, Faulds D, Sorkin EM. Inhaled fluticasone propionate: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma. Drugs 1994; 47(2): 318–31
Jarvis B, Faulds D. Inhaled fluticasone propionate: a review of its therapeutic efficacy at dosages ≤500 μg/day in adults and adolescents with mild to moderate asthma. Drugs 1999 May; 57(5): 769–803
Brogden RN, Faulds D. Salmeterol xinafoate: a review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs 1991; 42(5): 895–912
Adkins JC, McTavish D. Salmeterol: a review of its pharmacological properties and clinical efficacy in the management of children with asthma. Drugs 1997 Aug; 54(2): 331–54
Markham A, Jarvis B. Inhaled salmeterol/fluticasone propionate combination: a review of its use in persistent asthma. Drugs 2000 Nov; 60(5): 1207–33
Spencer CM, Jarvis B. Salmeterol/fluticasone propionate combination. Drugs 1999 Jun; 57(6): 933–40
Hanania NA, Ramsdell J, Payne K, et al., on behalf of the COPD Study Group. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50μg and fluticasone propionate 250μg alone or in combination via the Diskus [abstract]. Am J Respir Crit Care Med 2001 Apr; 163 (5 Suppl. 2): A279
Mahler DA, Wong E, Giessel G, et al. Improvements in FEV1 and symptoms in COPD patients following 24 weeks of twice daily treatment with salmeterol 50/fluticasone propionate 500 combination [abstract]. Am J Respir Crit Care Med 2001 Apr; 163 (5 Suppl. 2): A279
Wire P, Yates J, Fischer T, et al. The combination of fluticasone propionate and salmeterol administered with a single Diskus is safe and effective twice-daily maintenance treatment for COPD [abstract]. Chest 2001 Oct; 120 Suppl.: 162S–3S
Edin HM, Price MJ, Davis SL, et al. Combination salmeterol/fluticasone propionate administered by Diskus improves quality of life in patients with chronic obstructive pulmonary disease (COPD) [abstract]. Chest 2001 Oct; 120(4 Suppl.): 161S–2S
Edin HM, Price MJ, Wire P, et al. Fluticasone propionate/salmeterol combination improves quality of life in patients with COPD [abstract]. Am J Respir Crit Care Med 2001 Apr; 163 (5 Suppl. 2): A506
Calverley PMA, Pauwels RA, Vestbo J, et al., on behalf of the TRISTAN Study Group. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components in COPD [abstract]. Am J Respir Crit Care Med 2002; 165(8 Suppl.): A226
Jones PW, Edin HM, Anderson J. Salmeterol/fluticasone propionate combination improves health status in COPD patients [abstract]. Am J Crit Care Med 2002 Apr; 165(8 Suppl.): A111
GlaxoSmithKline. FDA Advisory Committee briefing document for use of Flovent Diskus and Advair Diskus in COPD [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/02/briefing/383061_04_FDA-ADVAIR.pdf [Accessed 2002 Apr 9]
Dowling RB, Johnson M, Cole PJ, et al. Effect of fluticasone propionate and salmeterol on Pseudomonas aeruginosa infection of the respiratory mucosa in vitro. Eur Respir J 1999; 14: 363–9
Pang L, Knox AJ. Synergistic inhibition by β2-agonists and corticosteroids on tumor necrosis factor-α-induced interleukin-8 release from cultured human airway smooth-muscle cells. Am J Respir Cell Molec Biol 2000 Jul; 23(1): 79–85
Pang L, Knox AJ. Regulation of TNF-α-induced eotaxin release from cultured human airway smooth muscle cells by β2-agonists and corticosteroids. FASEB J 2001 Jan; 15: 261–9
Baraniuk JN, Ali M, Brody D, et al. Glucocorticosteroids induce β2-adrenergic receptor function in human nasal mucosa. Am J Respir Crit Care Med 1997; 155: 704–10
Mak JCW, Nishikawa M, Shirasaki H, et al. Protective effects of glucocorticoid on downregulation of pulmonary β2-adrenergic receptors in vivo. J Clin Invest 1995; 96: 99–106
Eickelberg O, Roth M, Lörx R, et al. Ligand-independent activation of the glucocorticoid receptor by β2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem 1999 Jan 8; 274(2): 1005–10
Proud D, Reynolds CJ, Lichtenstein LM, et al. Intranasal salmeterol inhibits allergen-induced vascular permeability but not mast cell activation or cellular infiltration. Clin Exp Allergy 1998; 28: 868–75
Oddera S, Silvestri M, Scarso L, et al. Salmeterol inhibits the allergen-induced mononuclear cell proliferation and downregulates GM-CSF release and HLA-DR expression by monocytes. Pulm Pharmacol Ther 1997; 10: 43–9
Sekut L, Champion BR, Page K, et al. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin Exp Immunol 1995; 99(3): 461–6
Chambers CB, Corrigan BW, Newhouse MT. Salmeterol(s) speeds mucociliary transport (MCT) in healthy subjects [abstract]. Am J Respir Crit Care Med 1999; 159 (3 Suppl. 2): 636
Tay HL, Armoogum N, Tan LKS. Nasal mucociliary clearance and salmeterol. Clin Otolaryngol 1997; 22: 68–70
Kanthakumar K, Cundell DR, Johnson M, et al. Effect of salmeterol on human nasal epithelial cell ciliary beating: inhibition of the ciliotoxin, pyocyanin. Br J Pharmacol 1994; 112: 493–8
Dowling RB, Rayner CFJ, Rutman A, et al. Effect of salmeterol on Pseudomonas aeruginosa infection of respiratory muscosa. Am J Respir Crit Care Med 1997; 155: 327–36
Whelan CJ, Johnson M, Vardey CJ. Comparison of the anti-inflammatory properties of formoterol, salbutamol and salmeterol in guinea-pig skin and lung. Br J Pharmacol 1993; 110: 613–8
Bloemen PGM, van den Tweel MC, Henricks PAJ, et al. Increased cAMP levels in stimulated neutrophils inhibit their adhesion to human bronchial epithelial cells. Am J Phys 1997; 272 (4 Pt 1): 580–7
Ottonello L, Morone P, Dapino P, et al. Inhibitory effect of salmeterol on the respiratory burst of adherent human neutrophils. Clin Exp Immunol 1996; 106: 97–102
Nials AT, Coleman RA, Johnson M, et al. The duration of action of non-β2-adrenoceptor mediated responses to salmeterol. Br J Pharmacol 1997; 120: 961–7
Hattotuwa KL, Gizycki MJ, Ansari TW, et al. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med 2002; 165: 1592–6
Verhoeven GT, Hegmans JPJJ, Mulder PGH, et al. Effect of an inhaled glucocorticoid, fluticasone propionate (FP), on inflammation in bronchial biopsies of COPD patients with bronchial hyperresponsiveness (BHR) [abstract]. Am J Respir Crit Care Med 1999 Mar; 159 (3 Suppl. 2): A524
Yildiz F, Kaur AC, Ilgazli A, et al. Inhaled corticosteroids may reduce neutrophilic inflammation in patients with stable chronic obstructive pulmonary disease. Respiration 2000; 67: 71–6
Schoonbrood DFM, Out TA, Lutter R, et al. Plasma protein leakage and local secretion of proteins assessed in sputum in asthma and COPD: the effect of inhaled corticosteroids. Clin Chim Acta 1995; 240(2): 163–78
Man Y, West MR, Matthews JL, et al. Fluticasone propionate protects against viral infection of bronchial epithelial cells [abstract]. Am J Respir Crit Care Med 2001 Apr; 163 (5 Suppl. 2): A737
Dahl R. Salmeterol and fluticasone propionate in the treatment of asthma symptoms. Eur Resp Rev 1997; 7(49): 338–43
Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. J Allergy Clin Immunol 1998 Apr; 101 (4 Suppl. 2): 434–9
Kirby S, Falcoz C, Daniel MJ, et al. Salmeterol and fluticasone propionate given as a combination: lack of systemic pharmacodynamic and pharmacokinetic interactions. Eur J Clin Pharmacol 2001; 56: 781–91
Glaxo Wellcome Inc. Advair Diskus Product Information. Research Triangle Park (NC): Glaxo Wellcome Inc., 2000 Aug [online]. Available from URL: http://www.gsk.com/products/assets/us_advair.pdf [Accessed 2002 Apr 8]
Singh SD, Whale C, Hatton A, et al. Comparison of the pharmacokinetics of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease and healthy subjects. Am J Rep Crit Care Med 2001; 163(5): A281
Mehta R, Barnett V, Kunka RL, et al. Population pharmacokinetics (PK) of fluticasone propionate (FP) in subjects with asthma, COPD and healthy subjects [abstract no. TPII-83]. Clin Pharmacol Ther 2002 Feb; 71(1): P64
Mehta R, Barnett V, Daley-Yates PT, et al. Population pharmacokinetics (PK) of fluticasone propionate (FP) in subjects with asthma, COPD and healthy subjects [poster no. TPII-83]. 2002 Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, 2002; Atlanta (GA)
Chavannes NH, van Schayck CP. Quality of life in patients with chronic obstructive pulmonary disease: which drugs help most? Biodrugs 2000 Feb; 13(2): 127–33
Rutten-van Mölken M, Roos B, Van Noord JA. An empirical comparison of the St George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) in a clinical trial setting. Thorax 1999; 54: 995–1003
Ferguson G, Funck-Brenatano C, Fischer T, et al. Cardiovascular safety of salmeterol in patients with COPD [abstract]. Am J Resp Crit Care Med 2002 Apr; 165(8): A228
Lipworth BJ, Jackson CM. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Saf 2000 Jul; 23(1): 11–33
Zimmerman B, Gold M, Wherrett D, et al. Adrenal suppression in two patients with asthma treated with low doses of the inhaled steroid fluticasone propionate. J Allergy Clin Immunol 1998 Mar; 101(3): 425–6
GlaxoSmithKline Inc. The TORCH (TOwards a Revolution in COPD Health). Research Triangle Park (NC): GlaxoSmithKline Inc, 2002
Li JTC, Ford LB, Chervinsky P, et al. Fluticasone propionate powder and lack of clinically significant effects on hypothalamic-pituitary-adrenal axis and bone mineral density over 2 years in adults with mild asthma. J Allergy Clin Immunol 1999 Jun; 103(6): 1062–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A., Keating, G.M. Inhaled Salmeterol/Fluticasone Propionate Combination in Chronic Obstructive Pulmonary Disease. Am J Respir Med 1, 273–282 (2002). https://doi.org/10.1007/BF03256618
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256618