Summary
The prediction performances of population pharmacokinetic-pharmacodynamic analysis of the two methods (a stepwise and a simultaneous estimations) were evaluated with respect to their accuracies and precisions.
A study was designed to investigate the safety and efficacy of TS-943 by a 4 hours constant infusion in 36 healthy male subjects. Population analysis was performed using pharmacokinetic and pharmacodynamic models with NONMEM.
THe mean of the prediction error (MPE) and the root mean squared error (RMSE) served as a measure of accuracy and precision. In addition, a bootstrap validation was also performed.
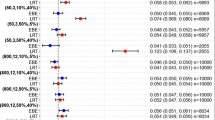
The results indicate that those population pharmacokinetic-pharmacodynamic parameters for the two methods were comparable. The results of simultaneous estimations are similar to those obtained using a stepwise estimation.
The mean parameter estimates obtained with the additional 200 bootstrap replicates of data were within 15% of those obtained with the final model in both methods. The present results demonstrated that the accuracy of pharmacodynamic evaluations using a stepwise end a simultaneous estimations was comparable.
Similar content being viewed by others
References
Furuya A., Kato N., Jingu S., Akimoto M., Higuchi S., Suwa T. and Ogata H. (2000): Population pharmacokinetics and pharmacodynamics of TS-943 for selective non-peptide platelet glycoprotein-IIb/IIIa receptor antagonist in normal healthy subjects. Clin. Pharmacol. Ther. 67:429–453.
Barrett J.S., Murphy G. et al., (1994): Pharmacodynamics and pharmacokinetics of MK-383, selective non-peptide platelet glycoprotein-IIb/IIIa receptor antagonist, in healty men Clin. Pharmacol. Ther; 56:377–388.
Ette. I.E. (1997): Stability and perfomance of a population pharmacokinetic model. J. Clin. Pharmacol. 37:486–495:
Sheiner, L.B, and Beal S.L. (1981): Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 9:503–512.
Beal S.L., Sheiner L.B. (1989): NONMEM users guide. San Francisco: NONMEM Project Group, University of California.
Takama H., Tanaka H., Sudo T., Tamura T., Tanigawara Y. (2001): Population pharmacokinetic modeling and model validation of a spicamycin derivative, KRN5500, in phase 1 study. Cancer Chemother Pharmacol. 47:404–10.
Van Warmerdam L.J., ten Bokkel Huinink W.W., Maes R.A., Beijnen J.H. (1994): Limited-sampling models for anticancer agents. J Cancer Res Clin Oncol; 120:427–33.
Hussei Z., Samara E., Locke C.S., Orcard M.A., Ringham G.L. and Granneman G.R. (1994): Characterization oh the pharmacokinetics and pharmacodynamics of a new pral thromboxane A2-receptor antagonist AA-2414 in normal subjects: Population analysis. Clin Pharmacol Ther;55:441–55.
Troconiz I.F., Naukkarinen T.H., Ruottinen H.M., Rinne U.K., Gordin A. and Karlsson M.O. (1998): Population pharmacodynamic modeling of levodopa in patients with parkinson’s disease receiving entacapone. Clin Pharmacol Ther; 64:106–16.
Marino M.T., Shuster B.G., Brueckner R.P., Lin E., Kaminskis A. and Lasseter K.C. (1998): Population pharmacokinetics and pharmacodynamics o pyridostigmine bromide for prophylaxia against nerve agents in humans. J Clin Pharmacol; 38:227–35.
Deschamps C., Dubruc C., Mentre F. and Rosenzweig P. (2000): Pharmacokinetic and pharmacodynamic modeling of mizolastine in healthy volunteers with an indirect response model. Clin Pharmacol Ther;68:647–57.
Parke J., Charles B.J. (2000): Factors affecting oral cyclosporin disposition after heart transplantation: bootstrap validation of a population pharmacokinetic models. Eur J Pharmacol;56:481–87.
Schwartz J.B., Verotta D., Sheiner L.B. Pharmacodynamic modeling of verapamil effects under steady-state and nonsteady-state conditions. J Pharmacol Exp Ther;251(3):1032–1038(1998).
Bellissant E., Sebille V., Paintaud G. (1998): Methodological issues in pharmacokinetic-pharmacodynamic modelling. Clin Pharmacokinet. 35(2):151–166.
Hashimoto Y., Sheiner LB. (1991): Designs for population pharmacodynamics: value of pharmacokinetic data and population analysis. J Pharmacokinet Biopharm 19(3):333–53.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Furuya, A., Kato, N., Jingu, S. et al. Comparison of stepwise and simultaneous estimations of population pharmacokinetics and pharmacodynamics of TS-943. Eur. J. Drug Metab. Pharmacokinet. 28, 191–199 (2003). https://doi.org/10.1007/BF03190485
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190485