Summary
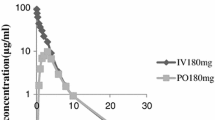
14C-piperacillin (Pipracil®) at a dose level of 1000 mg/kg was administered intravenously or intraperitoneally to pregnant and non-pregnant female rats. Rats were sacrificed at predetermined time intervals, and14C-piperacillin concentrations determined in serum, amniotic fluid, placenta, and fetus. The piperacillin serum levels in both pregnant and nonpregnant rats following intravenous administration were comparable; however, following intraperitoneal administration, the serum drug levels in the pregnant rats as compared to the nonpregnant rats were lower. The fetal drug levels, however, were higher following intraperitoneal administration than following intravenous administration. The total area under the fetal drug concentration-time curve following intraperitoneal administration was about two times the corresponding area under the curve following intravenous administration. The data indicate that the fetal exposure to piperacillin was greater following intraperitoneal administration.
Similar content being viewed by others
References
Kuck, N.A. and Redin, G.S. (1978): In-vitro and in-vivo activity of piperacillin, a new broad-spectrum semisynthetic penicillin. J. Amtibiot.,31, 1175–1182.
Sedman, A.J. and Wagner, J.G. (1974): A decision-making pharmacokinetic computer program. Univ. of Michigan, Michigan 48104.
Berman, M. and Weiss, M. (1977): User’s manual for SAAM27. National Institute, Bethesda, Maryland 20014.
Batra, V.K.:, Morrison, J.A., Lasseter, K.C., and Joy, V.A. (1979): Piperacillin kinetics. Clin. Pharmacol. Ther.,26, 41–53.
Saikawa, I., Takai, A., Nakashima, Y., Yoshida, C., Yasuda, T., Shimizu, E., Sakai, H., Taki, H., Tai, M., and Takashita, Y. (1977): Studies on β-lactam antibiotics for medicinal purpose VI. Studies on the metabolism of 6-[D(−)-α-(4-ethyl-2,3-dioxo-l-piperazine-carboxamido)-phenylacetamido]pencillanic acid (T-1220). J. Pharm. Soc. Jap.,97, 1071–1081.
Saikawa, I., Yasuda, T., Hideo, T., Watanabe, Y., Matsubara, N., Nakagawa, M., and Kanagawa, S. (1977): Absorption, excretion and tissue distribution of T-1220. Chemotherapy,25, 801–809.
Kramer, B., and Kramer, F. (1977): Drug kinetics in pregnancy. Clin. Pharmacokin.,2, 167–181.
Philipson, A. (1977): Pharmacokinetics of ampicillin during pregnancy. J. Inf. Dis.,136, 370–376.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Batra, V., Morrison, J. & Kaleita, E. Comparative pharmacokinetics of14C-piperacillin following intravenous and intraperitoneal administration in pregnant and non-pregnant rats. European Journal of Drug Metabolism and Pharmacokinetics 9, 31–39 (1984). https://doi.org/10.1007/BF03189603
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189603