Summary
The effect of food on the oral bioavailability of a manidipine 20 mg tablet was studied after a single administration in 12 male healthy subjects. The clinical trial was conducted as an open, randomised, crossover study. In two different administration sessions, the subjects received a 20 mg manidipine tablet either in the fasting state or after a standardized breakfast. Plasma samples were collected before and at different times after each administration for up to 24 h. The concentrations of manidipine and its pyridine metabolite (M-XIII metabolite) were determined by HPLC with coulometric detection.
The tolerability of manidipine was good. Only two cases of mild headache, one with each treatment, were reported.
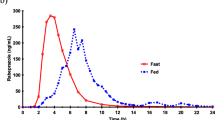
Food significantly improved the absorption, with an increase in AUC from 19.1 to 27.2 ng.h/ml (geometric mean, p<0.01) but did not modify the rate of absorption (tmax unchanged, median = 1.5 h). Peak plasma concentration was also increased (from 6.2 to 7.8 ng/ ml), but the difference was not statistically significant (p=0.18). Other pharmacokinetic parameters (apparent elimination half-life and mean residence time) remained unchanged.
The increase in bioavailability of manidipine administered with food is related to its high lipophilicity and may be explained through a solubilization effect produced by food and bile secretions.
Similar content being viewed by others
References
Iwata A., Tochikubo O., Kawano Y., et al. (1992): A study of the effects of manidipine on the diurnal variation of arterial pressure and hemodynamics in patients with essential hypertension. Blood Pressure, 1 (Suppl. 3): 87–93.
Mizuno K., Haga H., Takahaski M., et al. (1992): Clinical evaluation of the efficacy and safety of manidipine in hypertensive patients with renal disorders. Blood Pressure, 1 (Suppl. 3): 119–123.
Hirakata H., Iino K., Ishida I., et al. (1992): Effects of a new calcium antagonist, manidipine, on the renal hemodynamics and the vasoactive humoral factors in patients with diabetes mellitus. Blood Pressure, 1 (Suppl. 3), 124–129.
Morimoto S., Matsumura Y. (1991: Manidipine hydrochloride [CV-4093[2HCl)]. Cardiovas. Drugs Rev., 9: 207–222
Welling P.G. (1993: Necessity of food studies: implication of food effects. In: Midha K.K., Blume H.H. (eds.). Bio-International. Bioavailability, bioequivalence and pharmacokinetics, 92. Medpharm, Stuttgart, pp 211–221.
Tateno M. (1989): Pharmacokinetic study of CV-4093 (2HCl) at single dose on healthy subjects. J. Clin. Ther. Med., 5 (9): 1765–1790.
Gibaldi M., Perrier D. (1982): Pharmacokinetics, 2nd edition. Marcel Dekker, New York.
Fleiss J.L. (1986): The design and analysis of clinical experiments. Wiley Interscience, New York.
Steinijans V.W., Hauschke D. (1990: Update on the statistical analysis of bioequivalence studies. Int. J. Clin. Pharmacol. Ther. Toxicol. 28: 105–110.
Westlake W.J. (1988): Bioavailability and bioequivalence of pharmaceutical formulations. In: Peace KE (ed). Biopharmaceutical statistics for drug development. Marcel Dekker, New York, pp 329–352.
Steinijans V.W., Sauter R. (1993): Food studies: acceptance criteria and statistics. In: Midha K.K., Blume H.H. (eds). Bio-International. Bioavailability, bioequivalence and pharmacokinetics, 92. Medpharm, Stuttgart, pp. 335–250.
Chow S.C., Liu J.P. (1992): Design and analysis of bioavailability and bioequivalence studies. Marcel Dekker, New York.
Hauschke D., Steinijans V.W., Diletti E. (1990: A distribution-free procedure for the statistical analysis of bioequivalence studies. Int. J. Clin. Pharmacol. Ther. Toxicol., 28: 72–78.
Metzler C.M. (1988): Statistical methods for deciding bioequivalence of formulations. In: Yacobi A., Halperin-Walega E. (Eds). Oral sustained release formulations: Design and evaluation. Pergamon Press, New York, pp: 217–238.
Gibaldi M., (1991): Biopharmaceutics and Clinical Pharmacokinetics 4th Edition. Lea & Febiger.
Shimizu T., et al. (1993) Influence of food on the blood level and anti-hypertensive efficacy of calcium channel antagonist. Jpn J. Hosp. Pharm., 19 (6): 534–541.
Dunselman P.H.J.M., Edgar B. (1991): Felodipine clinical pharmacokinetics. Clin. Pharmacokinet., 21 (6): 418–430.
Ahnoff M., Persson B.A. (1990) Chromatography of calcium channel blockers. J. Chromatogr., 531: 181–213.
Challenor V.F., Waller D.G., Gruchy B.S., Renwick A.G., George C.F. (1987): Food and nifedipine pharmacokinetics. Br. J. Clin. Pharmacol., 23: 248–249.
Rietberg D.P., Love S.J., Zinny M. (1985): Effect of food on nifedipine pharmacokinetics. Clin. Pharmacol. Ther., 37: 223.
Rietberg D.P., Love S.J., Quercia G.T., Zinny M.A. (1987): Effect of food on nifedipine pharmacokinetics. Clin. Pharmacol. Ther., 42: 72–75.
Rimoy G.H., Idle J.R., Bhashar N.K., Rubin P.C. (1989): The influence of food on the pharmacokinetics of ‘biphasic’ nifedipine at steady state in normal subjects. Br. J. Clin. Pharmacol., 28: 612–615.
Ueno K., Kawashima S., Matsumoto K., Miyai K., Yamauchi K., Yamazaki K. (1991: Effect of light breakfast on the bioavailability of sustained release nifedipine. DICP- Ann. Pharmacotherapy, 25: 317–319.
Faulkner J.K., Hayden M.L., Chasseaud L.F., et al. (1989): Absorption of amlodipine unaffected by food: solid dose equivalent to solution dose. Arzneimittelforschung, 39: 799–801.
Abernethy D.R. (1989): The pharmacokinetic profile of amlodipine. Am. Heart. J., 118: 1100–1103.
Abernethy D.R. (1991: Amlodipine: pharmacokinetic profile of a low-clearance calcium antagonist. J. Cardiovasc. Pharmacol., 17: S4-S7.
Schran H.F., Jaffe J.M., Gonasum L.M. (1988): Clinical pharmacokinetics of isradipine. Am. J. Med., 84: 80–89.
Terakawa M., Tokuma Y., Shishido A., et al. (1987): Effect of two different meals on bioavailability of nilvadipine in healthy volunteers. J. Clin. Pharmacol., 27: 293–296.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosillon, D., Stockis, A., Poli, G. et al. Food effect on the oral bioavailability of Manidipine: single dose, randomized, crossover study in healthy male subjects. European Journal of Drug Metabolism and Pharmacokinetics 23, 197–202 (1998). https://doi.org/10.1007/BF03189339
Issue Date:
DOI: https://doi.org/10.1007/BF03189339