Abstract
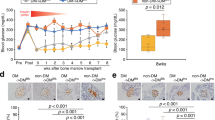
Curative therapy for diabetes mellitus mainly involves pancreas or islet transplantation to recruit insulin-producing cells. This approach is limited, however, because of both the shortage of donor organs and allograft rejection. Intra—bone marrow bone marrow transplantation (IBM-BMT) has recently been shown to be effective in inducing donor-specific tolerance in mice and rats without the use of immunosuppressants. After induction of diabetes in 15 C3H mice with streptozotocin, the mice received both allotransplants of bone marrow cells from C57BL/6 mice by IBM-BMT and injections via the portal vein of insulin-producing cells that were induced in vitro from stem cells derived from adult C57BL/6 bone marrow. We evaluated the expression of these cells by examining the expression of not only insulin but also the crucial transcription factors insulin I and insulin II. The diabetic mice were treated with IBM-BMT and precultured insulin-producing cells. Hyperglycemia was normalized by 5 days after the treatment and remained normal for more than 45 days. This strategy might be applicable to patients with type I diabetes mellitus.
Similar content being viewed by others
References
Powers AC. Diabetes mellitus. In: Brawnwald E, Fauci AS, Kasper DL, Hauser S, Longo DL, Jameson JL, eds. Harrison’sPrinciples of Internal Medicine. Vol. 2. New York, NY: McGraw-Hill; 2001:2109–2138.
Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen.N Engl J Med. 2000;343:230–238.
Gunnarsson R, Klintmalm G, Lundgren G, Wilczek H, Ostman J, Groth CG. Deterioration in glucose metabolism in pancreatic transplantation recipients given cyclosporine.Lancet. 1983;2:571–572.
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets.Science. 2001;292:1389–1394.
Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells.Proc Natl Acad Sci USA. 2002;99:16105–16110.
Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue.Proc Natl Acad Sci USA. 2000;97:7999–8004.
Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells.Nat Med. 2000;6:278–282.
Yang L, Li S, Hatch H, et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells.Proc Natl Acad Sci USA. 2002;99:8078–8083.
Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brain.Proc Natl Acad Sci USA. 1999;96:10711–10716.
Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis.Science. 1997;275:964–967.
Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization.Circ Res. 1999;85:221–228.
Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors.Science. 1998;279:1528–1530.
Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation.Nature. 1999;401:390–394.
Pereira RF, Halford KW, O’Hara MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice.Proc Natl Acad Sci USA. 1995;92:4857–4861.
Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. JClin Invest. 2002;109:1291–1302.
Moristot C, de Fraipont F, Richard MJ, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro.Stem Cells. 2005;23:594–603.
Tang DQ, Cao LZ, Burkhardt BR, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow.Diabetes. 2004;53:1721–1732.
Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes.Lab Invest. 2004;84:607–617.
Choi KS, Shin JS, Lee JJ, Kim YS, Kim SB, Kim CW. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract.Biochem Biophys Res Commun. 2005;330:1299–1305.
Brendel M, Hering B, Schultz A. International Islet Transplant Registry report.Int Transplant Regist Newslett. 2001;8:1–20.
Iwai H, Yasumizu R, Sugiura K, et al. Successful pancreatic allografts in combination with bone marrow transplantation in mice.Immunology. 1987;62:457–462.
Kaneda H, Adachi Y, Saito Y, et al. Long-term observation after simultaneous lung and intra-bone marrow-bone marrow transplantation. JHeart Lung Transplant. 2005;24:1415–1423.
Kushida T, Inaba M, Hisha H, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice.Blood. 2001;97:3292–3299.
Yasumizu R, Sugiura K, Iwai H, et al. Treatment of type 1 diabetes mellitus in non-obese diabetic mice by transplantation of allogeneic bone marrow and pancreatic tissue.Proc Natl Acad Sci USA. 1987;84:6555–6557.
Ikebukuro K, Adachi Y, Yamada Y, et al. Treatment of streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone marrow cells via portal vein in rats.Transplantation. 2002;73:512–518.
Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy.Circulation. 2005;112:1128–1135.
Cras-Méneur C, Elghazi L, Czernichow P, Scharfmann R. Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation.Diabetes. 2001;50:1571–1579.
Miettinen PJ, Huotari M, Koivisto T, et al. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors.Development. 2000;127:2617–2627.
Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of newly identified growth factor specific for epithelial cells.Proc Natl Acad Sci USA. 1989;86:802–806.
Yi ES, Yin S, Harclerode DL, et al. Keratinocyte growth factor induces pancreatic ductal epithelial proliferation.Am J Pathol. 1994;145:80–85.
Massagué J, Chen YG. Controlling TGF-β signaling.Genes Dev. 2000;14:627–644.
Kim SK, Hebrok M, Li E, et al. Activin receptor patterning of foregut organogenesis.Genes Dev. 2000;14:1866–1871.
Yamaoka T, Idehara C, Yano M, et al. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. JClin Invest. 1998;102:294–301.
Taira M, Inaba M, Takada K, et al. Treatment of streptozotocin-induced diabetes mellitus in rats by transplantation of islet cells from two major histocompatibility complex disparate rats in combination with intra bone marrow injection of allogeneic bone marrow cells.Transplantation. 2005;79:680–687.
Esumi T, Inaba M, Ichioka N, Kushida T, Iida H, Ikehara S. Successful allogeneic leg transplantation in rats in conjunction with intra-bone marrow injection of donor bone marrow cells.Transplantation. 2003;76:1543–1548.
Nakamura K, Inaba M, Sugiura K, et al. Enhancement of allogeneic hematopoietic stem cell engraftment and prevention of graft-versus-host diseases (GvHD) by intra-bone marrow bone marrow transplantation plus donor lymphocyte infusion.Stem Cells. 2004;22:125–134.
Fukui J, Inaba M, Ueda Y, et al. Prevention of graft-versus-host disease by intra-bone marrow injection of donor T cells.Stem Cells. 2007;25:1595–1601.
Ikehara S, Ohtsuki H, Good RA, et al. Prevention of type I diabetes in nonobese diabetic mice by allogeneic bone marrow transplantation.Proc Natl Acad Sci USA. 1985;82:7743–7747.
Ikehara S, Kawamura M, Takao F, et al. Organ-specific and systemic autoimmune diseases originate from defects in hematopoietic stem cells.Proc Natl Acad Sci USA. 1990;87:8341–8344.
Takao F, Yasumizu R, Than S, et al. Development of insulin-dependent diabetes mellitus in [(NOD + BALB/c)? NOD] mixed allogeneic bone marrow chimeras.Immunobiology. 1995;194:376–389.
Nathan DM. Long-term complications of diabetes mellitus.N Engl J Med. 1993;328:1676–1685.
Keymeulen B, Ling Z, Gorus FK. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft.Diabetologia. 1998;41:452–459.
Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy.Arch Histol Cytol. 2003;66:163–174.
Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration.Nat Biotechnol. 2003;21:763–770.
Verfaillie CM, Schwartz R, Reyes M, Jiang Y. Unexpected potential of adult stem cells.Ann N Y Acad Sci. 2003;996:231–234.
Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extrapancreatic insulin-producing cells in multiple organs in diabetes.Proc Natl Acad Sci USA. 2004;101:2458–2463.
Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. JClin Invest. 2003;111:843–850.
Topp BG, Mc Arthur MD, Finegood DT. Metabolic adaptations to chronic glucose infusion in rats.Diabetologia. 2004;47:1602–1610.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, M., Inaba, M., Guo, K.Q. et al. Treatment of streptozotocin-induced diabetes mellitus in mice by intra—bone marrow bone marrow transplantation plus portal vein injection of β cells induced from bone marrow cells. Int J Hematol 86, 438–445 (2007). https://doi.org/10.1007/BF02984002
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02984002