Summary
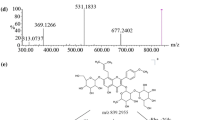
To study the metabolism of dauricine in vivo and in vitro and identify the structure of its main metabolites, urine of rats after drug administration as the samples of dauricine metabolism in vivo was studied. Rat liver S9 fraction was prepared and the oxygenation metabolism system reconstituted to perform phase I reaction of dauricine in vitro. TLC, HPLC-DAD and MS were used to analyze and identify dauricine and its main phase I metabolites in the samples. The results showed that besides the untransformed dauricine, in the urine samples there was little product of X’ which had the same features of TLC, HPLC-DAD and MS as those of N-desmethyl dauricine (N-ddau). Part of dauricine could be transformed to a main metabolite X after incubating with S9 fraction in appropriate conditions. The molecular ion peak of X was m/z 611. The full scan MS2 spectrum of m/z 611 peak from S9 sample were m/z 580, m/z 566, m/z 552, m/z 206, which were same as those of N-ddau. Liver is the major organ for dauricine metabolism and part of dauricine is biotransformed by liver. The major metabolite is considered to be N-ddau.
Similar content being viewed by others
References
1992. 26–38
1994.
Vickers S.In vivo andin vitro metabolism studies on a classI antiarrhythmic agent. Drug Metab Dispo, 1993, 21(3):467
Joerh L. A rapidin vitro method for the evaluation of potential antitumor drugs requiring metabolic activation by hepatic S9 enzymes. Biochem Pharmacol, 1989, 38(24): 4477
Chenery R J. The interaction of Omeprazole with rat liver cytochrome P450-mediated monooxygenase reactions in vitro and in vivo. Biochem Pharmacol, 1988, 37(7): 1407
1992, 27: 788
N- 1999, 15:12
Sanaullah, Bowers L D. Facile synthesis of [16, 16, 17- 2H3]-testosterone,-epitestosterone and their glucuronides and sulfates. J Steroid Biochem Mol Biol, 1996, 58(2):225
Raynaud F I, Mistry P, Donaghue Aet al. Biotransformation of the platinum drug JM216 following oral administration to cancer patients. Cancer Chemother Pharmacol, 1996, 38(2):155
Author information
Authors and Affiliations
Additional information
This project was suported by a grant from the foundation of Youth Sciences and Technology Chenguang Planning of Wuhan (No. 20005004033)
Rights and permissions
About this article
Cite this article
Shujuan, C., Li, L., Yimei, Y. et al. Metabolism of dauricine and identification of its main metabolites. Current Medical Science 20, 253–256 (2000). https://doi.org/10.1007/BF02887006
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02887006