Abstract
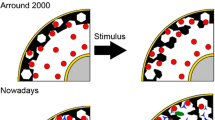
Earlier work by us as well as others has demonstrated that filamentous actin is mainly localized in the cortical surface of chromaffin cell. This F-actin network acts as a barrier to the chromaffin granules, impeding their contact with the plasma membrane. Chromaffin granules contain α-actinin, an anchorage protein that mediates F-actin association with these vesicles. Consequently, chromaffin granules crosslink and stabilize F-actin networks. Stimulation of chromaffin cell produces disassembly of F-actin and removal of the barrier. This interpretation is based on: (1) Cytochemical experiments with rhodamine-labeled phalloidin indicated that in resting chromaffin cells, the F-actin network is visualized as a strong cortical fluorescent ring; (2) Nicotinic receptor stimulation produced fragmentation of this fluorescent ring, leaving chromaffin cell cortical areas devoid of fluorescence; and (3) These changes are accompanied by a decrease in F-actin, a concomitant increase in G-actin, and a decrease in the F-actin associated with the chromaffin cell cytoskeleton (DNAse I assay). We also have demonstrated the presence in chromaffin cells of gelsolin and scinderin, two Ca2+-dependent actin filament-severing proteins, and suggested that chromaffin cell stimulation activates scinderin with the consequent disruption of F-actin networks. Scinderin, a protein recently isolated in our laboratory, is restricted to secretory cells and is present mainly in the cortical chromaffin cell cytoplasm. Scinderin, which is structurally different from gelsolin (different pIs, amino acid composition, peptide maps, and so on), decreases the viscosity of actin gels as a result of its F-actin-severing properties, as demonstrated by electron microscopy. Stimulation of chromaffin cells either by nicotine (10 μM) or high K+ (56 mM) produces a redistribution of subplasmalemmal scinderin and actin disassembly, which preceded exocytosis. The redistribution of scinderin and exocytosis is Ca2+-dependent and is not mediated by muscarinic receptors. Furthermore, our cytochemical experiments demonstrate that chromaffin cell stimulation produces a concomitant and similar redistribution of scinderin (fluorescein-labeled antibody) and F-actin (rhodamine phalloidin fluorescence), suggesting a functional interaction between these two proteins. Stimulation-induced redistribution of scinderin and F-actin disassembly would produce subplasmalemmal areas of decreased cytoplasmic viscosity and increased mobility for chromaffin granules. Exocytosis sites, evaluated by antidopamine-β-hydroxylase (anti-DβH) surface staining, are preferentially localized in plasma membrane areas devoid of F-actin.
Similar content being viewed by others
References
Aunis D. and Perrin D. (1984) Chromaffin granule membrane-F-actin interactions and spectrin-like protein of subcellular organelles: a possible relationship.J. Neurochem. 42, 1558–1569.
Aunis D., Guerold B., Bader M.-F. and Cieselski-Treska J. (1980) Immunocytochemical and biochemical demonstration of contractile proteins in chromaffin cells in culture.Neuroscience 5, 2261–2277.
Bader M. F. and Aunis D. (1983) The 97 kD α-actinin-like protein in chromaffin granule membranes from adrenal medulla, evidence for localization on the cytoplasmic surface and for binding to actin filaments.Neuroscience 8, 165–181.
Bader M.-F., Sontag J.-M., Thiersé D., and Aunis D. (1989) A reassessment of guanine nucleotide effects of catecholamine secretion from permeabilized adrenal chromaffin cells.J. Biol. Chem. 264, 16,426–16,434.
Bader M. F., Trifaró J.-M., Langley, O. K., Thiersé D., and Aunis D. (1986) Secretory cell actin-binding proteins, identification of a gelsolin-like protein in chromaffin cells.J. Cell Biol. 102, 636–646.
Bendayan M., Marceau N., Beaudoin, A. R., and Trifaró J.-M. (1982) Immunocytochemical localization of actin in the pancreatic exocrine cell.J. Histochem. Cytochem. 30, 1075–1078.
Bernstein F. W. and Bamburg J. R. (1985) Reorganization of actin in depolarized synaptosomes.J. Neurosci. 5, 2565–2569.
Birchmeier W. (1984) Cytoskeleton structure and function.Trends Biochem. Sci. 9, 192–195.
Buckley K. and Kelly R. B. (1985) Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells.J. Cell Biol. 100, 1284–1294.
Burgoyne R. D. and Cheek T. R. (1988) Role of chromaffin cell cytoskeleton in secretion.Progress in Catecholamine Research, Part A: Basic Aspects and Peripheral Mechanisms, Dahlstrom A., Belmaker R. H., and Sandler, M., eds., R. Liss, New York, pp. 253–256.
Burgoyne R. D., Cheek T. R., and Norman K. M. (1986) Identification of a secretory granule-binding protein as caldesmon.Nature 319, 68–70.
Burgoyne R. D., Morgan A., and O'Sullivan A. J. (1989) The control of cytoskeletal actin and exocytosis in intact and permeabilized adrenal chromaffin cells: role of calcium and protein kinase C.Cell. Signal. 1, 323–334.
Cheek T. R. and Burgoyne R. D. (1986) Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells.FEBS Lett. 207, 110–114.
Cheek T. R. and Burgoyne R. D. (1987) cAMP inhibits both nicotine-induced actin disassembly and catecholamine secretion from bovine adrenal chromaffin cells.J. Biol. Chem. 262, 11,663–11,666.
Cheek T. R., Jackson F. R., O'Sullivan A. J., Moreto R. B., Berridge M. J., and Burgoyne R. D. (1989) Simultaneous measurements of cytosolic Ca2+ and secretion in single bovine adrenal chromaffin cells by fluorescent imaging of fura-2 in co-cultured cell.J. Cell Biol. 109, 1219–1227.
De Robertis E. D. P. and Bennett H. S. (1954) Submicroscopic vesicular component in the synapse.Fed. Proc. 13, 35.
De Robertis E. D. P. and Bennett H. S. (1955) Some features of the submicroscopic morphology of synapses in frog and earthworm.J. Bioph. Bioch. Cytol. 1, 47–58.
De Robertis E. D. P. and Vaz Ferreira A. (1957) Electron microscopic study of the excretion of catechol containing droplets in the adrenal medulla.Exp. Cell Res. 12, 568–574.
Douglas W. W. (1968) Stimulus-secretion coupling: The concept and clues from chromaffin and other cells.Br. J. Pharmacol. 34, 451–474.
Douglas W. W. and Rubin R. P. (1961) The role of calcium in the secretory response of the adrenal medulla to acetylcholine.J. Physiol. 159, 40–57.
Fisher S. K., Holz R. W., and Agranoff B. W. (1981) Muscarine receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion.J. Neurochem. 37, 491–497.
Green R. and Shields D. (1984) Somatostatin discriminates between the intracellular pathways of secretory and membrane proteins.J. Cell Biol. 99, 97–104.
Gumbiner B. and Kelly R. B. (1982) Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells.Cell 28, 51–59.
Harvey D. G. and MacIntoch F. C. (1940) Calcium and synaptic transmission in a sympathetic ganglion.J. Physiol. 97, 408–418.
Houssay B. A. and Molinelli, E. A. (1928) Excitabilité des fibres adrénalino-sécrétories du neuf grand splanchnique: fréquences, seuil et optimum des stimulus: rôle de l'ion calcium.C. R. Seances Soc. Biol. Ses Fil. 99, 172–174.
Hughes A. R. and Putney J. W. Jr. (1990) Inositol phosphate formation and its relationship to calcium signals.Environ. Health Persp 84, 141–147.
Joh T. H. and Hwang O. (1987) Dopamine-β-hydroxylase, biochemistry and molecular biology.Ann. N. Y. Acad. Sci. 493, 343–350
Kao L.-S. and Schneider A. S. (1985) Muscarine receptors on bovine chromaffin cells mediate a rise in cytosolic calcium that is independent of extracellular calcium.J. Biol. Chem. 260, 2019–2022.
Kelly R. B. (1985) Pathways of protein secretion in eukaryotes.Science 230, 25–31.
Kenigsberg R. L. and Trifaró J.-M. (1980) Presence of high affinity uptake, system for catecholamines in cultured bovine adrenal chromaffin cells.Neuroscience 5, 1547–1556.
Kenigsberg R. L. and Trifaró J.-M. (1985) Microin-jections of calmodulin antibodies into cultured chromaffin cells blocks catecholamine release in response to stimulation.Neuroscience 14, 335–337.
Kim K.-T. and Westhead E. W. (1989) Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2 and secretion from bovine chromaffin cells.Proc. Natl. Acad. Sci. USA 86, 9881–9885.
Koffer A., Tatham P. E. R., and Gompers B. D. (1990) Changes in the state of actin during the exocytotic reaction in mast cells.J. Cell Biol. 111, 919–927.
Kondo T. H., Wolosewick J. J., and Pappas G. D. (1982) The microtrabecular lattice of the adrenal medulla revealed by polyethylene glycol embedding and stereo electron microscopy.J. Neurosci 2, 57–65.
Kwiatkowski, D. J., Janmey P. A., Mole J. E., and Yin H. L. (1985) Isolation and properties of two actin-binding domains in gelsolin.J. Biol. Chem. 260, 15,232–15,238.
Lee R. W. H. and Trifaró J.-M. (1981) Characterization of anti-actin antibodies and their use in immunocytochemical studies on the localization of actin in adrenal chromaffin cells in culture.Neuroscience 6, 2087–2108.
Lee R. W. H., Mushynski W. E., and Trifaró J.-M. (1979) Two forms of cytoplasmic actin in adrenal chromaffin cells.Neuroscience 4, 843–852.
Matter K., Dreyer F., and Aktories K. (1989) Actin involvement in exocytosis from PC12 cells: studies on the influence of botulinun C2 toxin on stimulated noradrenaline release.J. Neurochem. 52, 370–376.
Perrin D. and Aunis D. (1985) Reorganization of fodrin induced by stimulation in secretory cells.Nature 315, 589–591.
Phillips J. M., Burridge K., Wilson S. O., and Kirshner N. (1983) Visualization of the exocytosis/endocytosis secretory cycle in cultured adrenal chromaffin cells.J. Cell Biol. 97, 1906–1917.
Rodríguez Del Castillo A., Lemaire S., Tchakarov L., Jeyapragasan M., Doucet J.-P., Vitale M. L., and Trifaró, J.-M. (1990) Chromaffin cell scinderin: a novel calcium-dependent actin-filament severing protein.EMBO J. 9, 43–52.
Rothman J. H., Lam C., and Stevens T. H. (1985) Protein sorting and organelle assembly in yeast,Protein Transport and Secretion: Current Communications in Molecular Biology, Gething M. J., ed., Cold Spring Harbor Laboratory, New York, pp. 190–195.
Smith A. D. (1968) The storage of hormones.Biochem. J. 109, 17–19.
Tartakoff A. M., Vassalli, P., and Detraz M. (1978) Comparative studies of intracellular transport of secretory proteins.J. Cell Biol. 79, 694–707.
Tchakarov L., Vitale M. L., Jeyapragasan M., Rodríguez Del Castillo A., and Trifaró J.-M. (1990) Expression of scinderin, an actin filament-severing protein, in different tissues.FEBS Lett. 268, 209–212.
Trifaró J.-M. (1977) Common mechanisms of hormone secretion.Annu. Rev. Pharmacol. Toxicol. 17, 27–47.
Trifaró J.-M. (1978) Contractile proteins in tissues originating in the neural crest.Neuroscience 3, 1–24.
Trifaró, J.-M. (1982) The cultured chromaffin cell: a model for the study of biology and pharmacology of paraneurons.Trends Pharmacol. Sci. 3, 389–392.
Trifaró, J.-M. (1984) The adrenal paraneuron, its biology and pharmacology.Can. J. Physiol. Pharmacol. 62, 465–466.
Trifaró, J.-M. (1990) The 1989 Upjohn Award Lecture: cellular and molecular mechanisms in hormone and neurotransmitter secretion.Can. J. Physiol. Pharmacol. 68, 1–16.
Trifaró J.-M., Bader M.-F., Côté A., Kenigsberg R. L., Hikita T., and Lee R. W. H. (1985a) Cytoskeleton organization and adrenal chromaffin cell function,Contractile Proteins in Muscle and Non Muscle Cell Systems, Alia E. E., Arena N., and Russo, M. A., eds., Praeger, New York, pp. 459–472.
Trifaró, J.-M., Bader M.-F., and Doucet J. P. (1985b) Chromaffin cell cytoskeleton: its possible role in secretion.Can. J. Biochem. Cell Biol. 63, 661–679.
Trifaró, J.-M. and Bourne G. W. (1981) Differential effects of concanavalin A on acetylcholine and potassium-evoked release of catecholamines from cultured chromaffin cells.Neuroscience 6, 1823–1833.
Trifaró, J.-M. and Fournier S. (1987) Calmodulin and the secretory vesicle.Ann. NY. Acad. Sci. 493, 417–434.
Trifaró, J.-M., Fournier S., and Doucet J.-P. (1988) Calmodulin and the cytoskeleton in secretion.Proc. Alfred Benzon Symp. 25, 632–654.
Trifaró, J.-M. Kenigsberg R. L., Côté A., Lee R. W. H., and Hikita T. (1984) Adrenal paraneuron contractile proteins and stimulus-secretion coupling.Can. J. Physiol. Pharmacol. 62, 493–501.
Trifaró J.-M. and Lee R. W. H. (1978)Catecholamines: Basic and Clinical Frontiers. Proceedings of the 4th International Catecholamines Symposium. Usdin E., Kopin J. J., and Barchas J., eds., Pergamon, New York, pp. 358–360.
Trifaró, J.-M. and Lee R. W. H. (1980) Morphological characteristics and stimulus-secretion coupling in bovine adrenal chromaffin cell cultures.Neuroscience 5, 1533–1546.
Trifaró, J.-M., Lee R. W. H., Kenigsberg R. L., and Côté A. (1982) Contractile proteins and chromaffin cell function.Adv. Biosci. 36, 151–158.
Trifaró, J.-M., Novas M. L., Fournier S., and Rodríguez Del Castillo A. (1989) Cellular and molecular mechanisms in hormone and neurotransmitter secretion,Recent Advances in Pharmacology and Therapeutics, Velasco M., Israel A., Romero E., and Silva H., eds., Elsevier, New York, pp. 15–20.
Trifaró J.-M. and Poisner A. M. (1982) Common properties in the mechanisms of synthesis, processing and storage of secretory productsThe Secretory Process, The Secretory Granule, Vol. 2, Poisner A. M. and Trifaró, J.-M., eds., Elsevier, North Holland, pp. 387–407.
Trifaró, J.-M. and Ulpian C. (1976) Isolation and characterization, of myosin from the adrenal medulla.Neuroscience 1, 483–488.
Vitale M. L., Rodríguez Del Castillo A., Tchakarov L., and Trifaró J.-M. (1991) Cortical filamentous actin disassembly and scinderin redistribution during chromaffin stimulation precede exocytosis, a phenomenon not exhibited by gelsolin.J. Cell Biol. 113, 1057–1067.
Vitale M. L., Rodríguez Del Castillo A., and Trifaró J.-M. (1991) Protein kinase C modulates scinderin redistribution and F-actin disassembly in chromaffin cells. 6th Int. Symp. on Chromaffin Cell Biology, August 18–23 Marburg, Germany.
Wilson S. P. and Kirshner, N. (1977) The acetylcholine receptor of the adrenal medulla.J. Neurochem. 28, 687–695.
Yin H. L. and Stossel T. P. (1979) Control of cytoplasmicactin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein.Nature 281, 583–586.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trifaró, J.M., Rodríguez Del Castillo, A. & Vitale, M.L. Dynamic changes in chromaffin cell cytoskeleton as prelude to exocytosis. Mol Neurobiol 6, 339–358 (1992). https://doi.org/10.1007/BF02757940
Issue Date:
DOI: https://doi.org/10.1007/BF02757940