Abstract
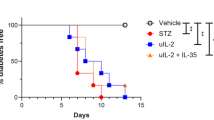
DLE was prepared from the minority of euglycemic CD-1 mice, previously injected with STZ, and was administered to hyperglycemic CD-1 male mice 1, 2 and 3 weeks after completion of multidose STZ. Mice treated with DLE derived from 2 × 107 (1X) or 108 lymphocyte equivalents (lymph.equ.) were significantly less hyperglycemic than the saline treated controls (P<0.001). The effects of DLE remained evident for more than 10 weeks after the final DLE treatment. Mice treated with DLE prepared from diabetic mice (hg DLE) developed a somewhat more rapid onset of hyperglycemia than the STZ treated control animals, although this effect did not achieve statistical significance (P=0.1). This DLE was absorbed on a rat insulinoma cell line (RIN), which contains interspecies cross-reacting islet antigens, and compared to the unabsorbed DLE. Mice treated with hg DLE preabsorbed on RIN cells, showed a slower onset of hyperglycemia. DLE prepared from euglycemia mice and the RIN- absorbed fraction were equally capable of preventing hyperglycemia (P<0.05).
In order to determine whether the DLE effects were genetically restricted, DLE was prepared from BALB/c mice, normally resistant to the diabetogenic effects of multidose STZ, both before and after STZ treatment. STZ primed CD-1 mice treated with 3 weekly doses of 2 × 107 lymph. equ. of untreated BALB/c derived DLE, STZ treated BALB/c derived DLE, and STZ treated CD-1 DLE were all less hyperglycemic than the control mice, who received saline (P<0.001). However, mice treated with CD-1 DLE were less hyperglycemic than the mice given BALB/c derived DLE (P<0.05). These effects were relatively long-lived.
Mice that were given the >3,500 Dalton fraction of CD-1 DLE were significantly less hyperglycemic than either the control mice or those treated with the <3,500 Dalton fraction of CD-1 DLE (P<0.05). Effects remained evident for more than 3 months after the last dose of DLE. Pancreatic tissue from the mice treated with the >3,500 Dalton fraction of CD-1 derived DLE revealed slightly more islets of a slightly greater size with less surrounding inflammation than either control mice or mice treated with the <3,500 Dalton fraction of DLE.
Similar content being viewed by others
Abbreviations
- ANOV:
-
analysis of variance
- DLE:
-
dialyzable lymphoid extract
- lymph.equ.:
-
lymphocyte equivalents
- RIN:
-
rat insulinoma cell line
- STZ:
-
streptozotocin
References
Gepts W & DeMay J. Islet cell survival determined by morphology. Diabetes 1978; 27(suppl.1): 251–56.
Nerups J, Andersen OO, Bendixen G et al.. Antipancreatic cellular hypersensitivity in diabetes mellitus. Diabetes 1971; 20: 424–27.
Lernmark A, Freedman ZR, Hoffman C et al. Islet cell surface antibodies in juvenile diabetes. N Engl J Med 1978; 299: 375–80.
Buschard K, Ropke C, Medsbad S, et al. Alterations of peripheral blood T-lymphocyte populations in patients with insulin-dependent (type I) diabetes mellitus. J Clin Lab 1983; 10: 127–31.
Buschard K, Ropke C, Medsbad S, et al. T lymphocyte subsets in patients with newly diagnosed type I (insulin-dependent) diabetes: a perspective study. Diabetology 1983; 25: 247–51.
Stiller CR, Dupre J, Gent M, et al. Effects of cyclosporine immunosuppression in insulin-dependant diabetes mellitus of recent onset. Science 1984; 223: 1362.
Rossini AA, Appel MC, Williams RM & Like AA. Genetic influence of the streptozotocin-induced insulitis and hyperglycemia. Diabetes 1977; 26: 916–20.
Rossini AA, Williams RM, Appel MC & Like AA. Sex differences in the multiple dose streptozotocin model of diabetes. Endocrinol 1978; 103: 1518–22.
Eisenbarth GS, Morris MA & Scearce RM. Cytotoxic antibodies to cloned rat islet cells in serum of patients with diabetes mellitus. J Clin Inv 1981; 67: 403–8.
Lawrence HS & Borkowsky W. A new basis for the immunoregulatory activities of transfer factor — an arcane dialect in the language of cells. Cell Immun 1983; 82: 102–16.
Borkowsky W & Lawrence HS. Antigen-specific suppressor factor in human leukocyte dialysates suppresses in vivo foot-pad reactivity in immunized BALB/C mice. In: CH Kirkparick, HS Lawrence, D Burger, eds. Transfer Factor: Fourth International Workshop. New York: Academic Press, 1983.
Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: a new model of diabetes mellitus. Science 1976; 193: 415–17.
Rossini A, Like A, Chick W, et al. Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci 1977; 74: 2485–89.
Appel MC, Rossini AA, Williams RM & Like AA. Viral studies in streptozotocin-induced pancreatic insulitis. Diabetolog 1978; 15: 327–36.
Herold KC, Montag AG & Fitch FW. Treatment with anti-T-lymphocyte antibodies prevents induction of insulitis in mice given multiple doses of streptozotocin. Diabetes 1987; 36: 796–801.
Paik SG, Fleischer N & Shin S-I. Insulin-dependent diabetes mellitus induced by subdiabetogenic doses of streptozotocin: Obligatory role of cell-mediated autoimmune processes. Proc Natl Acad Sci 1980; 77: 6129–33.
Buschard K & Rygaard J. Passive transfer of streptozotocin induced diabetes mellitus with spleen cells. Acta Pathol Microb Scand [c] 1977; 85: 469–72.
Blue ML & Shin SI. Diabetes induction by subdiabetogenic doses of streptozotocin in Balb,cBOM mice. Noninvolvement of host B- lymphocyte functions. Diabetes 1984; 33: 105–10.
Nakamura M, Nagafuchi S, Yamaguchi K & Takaki R. The role of thymic immunity and insulitis in the development of streptozotocin- induced diabetes in mice. Diabetes 1984; 33: 894
McEvoy RC, Andersson J, Sandler S & Hellerstrom C. Multiple low-dose streptozotocin-induced diabetes in the mouse. J Clin Inv 1984; 74: 715–22.
Kim WT & Steinberg C. Immunologic studies on the induction of diabetes in experimental animals. Cellular basis for the induction of diabetes by streptozotocin. Diabetes 1984; 33: 771–77.
Campbell IL, Oxbrow L, Koulmanda M & Harrison LC. IF-gamma induces islet cell MHC antigens and enhances autoimmune streptozotocin induced diabetes in the mouse. J Immunol 1988; 140: 1111–16.
Brummer E, Foster LG, Bhardwaj N & Lawrence HS. A murine model for studying the transfer of DTH with dialyzable human transfer factor. In: A Khan, C Kirkpatrick, NO Hill, eds. Immune Regulators in Transfer Factor. New York: Academic Press, 1979: 27–38.
Bieg S, Seissler J, Herberg L, et al. GAD65 is recognized by T cells, but not by antibodies from NOD-mice. Autoimmun 1994; 17: 189–94.
Bonifacio E & Boitard C. T cell reactivity to 38kD insulin-secretory granule protein in patients with recent onset type 1 diabetes. J Endoc Inv 1994; 17: 559–63.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borkowsky, W., Pilson, R. & Lawrence, H.S. Dialyzable lymphoid extract (DLE) from mice resistant to STZ-induced diabetogenesis can interrupt the progress of diabetes in STZ-treated CD-1 mice. Biotherapy 9, 149–157 (1996). https://doi.org/10.1007/BF02628673
Issue Date:
DOI: https://doi.org/10.1007/BF02628673