Abstract
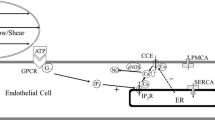
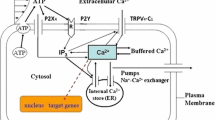
A mathematical model is proposed to describe the intracellularCa 2+ (Ca i) transient and electrical activity of vascular endothelial cells (VEC) elicited by fluid shear stress (τ). The intracellularCa 2+ store of the model VEC is comprised of aCa i-sensitive (sc) and an inositol (1,4,5)-trisphosphate (IP 3)-sensitive compartment (dc). The dc [Ca 2+] is refilled by the sc whose [Ca 2+] is the same as extracellular [Ca 2+].IP 3 produced by the τ-deformed mechanoreceptors discharges the dcCa 2+ into the cytosol. The increase of cytosolic[Ca 2+] inducesCa 2+ release (CICR) from the sc. The raisedCa i activates aCa i-activatedK + current (I K, Ca) and inhibitsIP 3 production. The cell membrane potential is determined byI K, Ca, voltage-dependentNa + andK + currents. Steady τ>0.1 dyne/cm2 elicits aCa i varies sigmoidally withLog 10(τ) with a maximal peakCa i of 150 nM at τ=4 dynes/cm2. Step increases of τ fail to elicit aCa 2+ response in cells previously stimulated by a lower shear. TheCa 2+ response gradually decreases with repetitive τ stimuli. Pulsatile shear elicits two to three times higherCa i and hyperpolarizes the cell more than steady shear of the same magnitude. The simulatedCa 2+ responses to τ are quantitatively and qualitatively similar to those observed in cultured VEC. The model provides a possible explanation of why the vasodilating stimulus is greater for pulsatile flow than for nonpulsatile flow.
Similar content being viewed by others
References
Ando, J., T. Komatsuda, and A. Kamiya. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cell.In Vitro Cell Dev. Biol. 24:871–877, 1988.
Ando, J., A. Ohtsuka, T. Kawamura, and A. Kimiya. Wall shear stress rather than shear rate regulates cytoplasmicCa ++ responses to flow in vascular endothelial cells.Biochem. Biophys. Res. Commun. 190:716–723, 1993.
Barbee, K. E., P. F. Davies, and R. Lal. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy.Circ. Res. 74:163–171, 1994.
Batty, I. R., S. R. Nahorsko, and R. F. Irvine. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices.Biochem. J. 232:211–215, 1985.
Cooke, J. P., J. Stamler, N. N. Andon, R. F. Davies, G. McKinle, and J. Loscalzo. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol.Am. J. Physiol. 259:H804-H812, 1990.
Davies, R. F., A. Remuzzi, E. J. Gordon, C. F. Dewey, Jr., and M. A. Grimbrone. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro.Proc. Natl. Acad. Sci. U.S.A. 83:2114–2117, 1986.
Davies, P. F., and S. C. Tripathi. Mechanical stress mechanisms and the cell. An endothelial paradigm.Circ. Res. 72:239–245, 1993.
Davies, P. F., and K. A. Barbee. Endothelial cell surface imaging: insight into hemodynamic force transduction.News Physiol. Sci. 9:153–157, 1994.
De Paola, N., M. A. Gimbrone, P. F. Davies, and C. F. Dewey. Vascular endothelium responds to fluid shear stress gradients.Arteriosclerosis Thromb. 12:1254–1257, 1992.
Dull, R. O., and R. F. Davies. Flow modulation of agonist (ATP)-response (Ca 2+) coupling in vascular endothelial cells.Am. J. Physiol. 261:H149-H154, 1991.
Frangos, J. A., S. G. Eskin, L. V. McIntire, and C. L. Ives. Flow effect on prostacyclin production by cultured human endothelial cells.Science Wash. D.C. 227:1477–1479 1985.
Giffith, T. M., and D. H. Edwards. Myogenic autoregulation of flow may be inversely related to endothelium-derived relaxing factor activity.Am. J. Physiol. 258:H1171-H1180, 1990.
Goldschmodt-Clermont, P. J., L. M. Mackesky, J. J. Baldassare, and T. D. Pollard. The actin-binding protein profilin binds toPIP 2 and inhibits its hydrolysis by phospholipase C.Science 247:1575–1578, 1990.
Gould, L. K.Coronary Artery Stenosis. New York Elservier Science, 1991. pp. 41–52.
Gould, L. K. Reversal of coronary atherosclerosis.Circulation 90:1558–1571, 1994.
Hensen, C. A., S. Mah, and J. R. William. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver.J. Biol. Chem. 261:8100–8103, 1986.
Holtz J., U. Forstermann, M. Pohl, M. Giesler, and E. Bassenge. Flow-dependent endothelium-mediated dilation of epicardial coronary arteries in conscious dog: effect of cyclooxygenase inhibition.J. Cardiovasc. Pharmacol. 6: 1161–1169, 1984.
Hutchesson, I. R., and T. M. Griffith. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow.Am. J. Physiol. 261:H257–262, 1991.
Irvine, R. F. How do inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate regulate intracellularCa 2+?Biochem. Soc. Trans. 17:6–8, 1989.
Kuo, L., M. J. Davies, and W. M. Chilian: Endothelium-dependent, flow-induced dilation of isolated coronary arteries.Am. J. Physiol. 259:H1063-H1070, 1990.
Lansman, J. B., T. J. Hallam, and T. J. Rink. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers.Nature 325:811–813, 1987.
Melkumyants, A. M., S. A. Belashov, E. S. Veselova, and V. Khayutin. Continuous control of the lumen of feline conduit arteries by blood flow rate.Cardiovasc. Res. 21: 863–870, 1987.
Mo, M., S. G. Eskin, and W. P. Schilling. Flow-induced changes inCa 2+ signalling of vascular endothelial cells: effect of shear stress and ATP.Am. J. Physiol. 260:H1698-H1707, 1991.
Nollert, M. V., S. G. Eskin, and L. V. McIntire. Shear stress increases inositol trisphosphate levels in human endothelial cells.Biochim. Biophys. Res. Commun. 170:281–287, 1990.
Oike, M., Droogmans, G., and Nilius B. MechanosensitiveCa 2+ transients in endothelial cells from human umbilical vein.Proc. Natl. Acad. Sci. U.S.A. 91:2940–2944, 1994.
Olesen, S. P., D. E. Clapham, and P. F. Davies. Heemodynamic shear stress activates aK + current in vascular endothelial cells.Nature 311:168–170, 1988.
Pohl, U., R. Busse, E. Kuo, and E. Bassenge. Pulsatile perfusion stimulates the release of endothelial autacoids.J. Appl. Cardiol. 1:215–235, 1986.
Rubanyi, G. M., J. C. Romero, and P. M. Vanhoutte. Flow-induced release of endothelium-derived relaxing factor.Am. J. Physiol. 250:H1145-H1149, 1986.
Sage, S. O., D. J. Adams, and C. van Breemen. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers.J. Biol. Chem. 264:6–9, 1989.
Sanchez-Bueno, A., C. J. Dixon, N. W. Woods, K. S. R. Cuthbertson, and P. H. Cobbold. Inhibitors of protein kinase C prolong the falling phase of each free-Ca transient in a hormone-stimulated hepatocyte.Biochem. J. 268:627–632, 1990.
Satcher, R. L. J., S. R. Bussolari, M. A. J. Gimbrone, and C. F. J. Dewey. The distribution of fluid forces on model arterial endothelium using computational fluid dynamics.J. Biomech. Eng. 114:309–316, 1992.
Smiesko, V. M., J. Kozik, and S. Dolezel. Role of endothelium in the control of arterial diameter by blood flow.Blood Vessels 22:247–251, 1985.
Treasure, C. B., J. A. Vita, D. A. Cox, R. D. Fish, J. B. Gordon, G. H. Mudge, W. S. Colucci, M. G. St John-Sutton, A. P. Selwyn, R. W. Alexander, and P. Ganz. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy.Circulation 81: 772–779, 1990.
Shen, J., F. W. Luscinskas, A. Connolly, C. F. Dewey, Jr., and M. A. Gimbrone. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells.Am. J. Physiol. 262:C384-C390, 1992.
Shen, J., F. W. Luscinskas, M. A. Gimbrone, Jr., and C. F. Dewey, Jr. Fluid flow modulates vascular endothelial cytosolic calcium responses to adenine nucleotides.Microcirculation 1:67–78, 1994.
Starmer, C. F. Characterizing activity-dependent processes with a piecewise exponential model.Biometrics 44:549–559, 1988.
Watson, P. A. Function follows form: generation of intracellular signal by cell deformation.FASEB J. 5:2013–2019, 1991.
Wong, A. Y. K., and G. A. Klassen. A model of cytosolic calcium regulation and autacoids production in vascular endothelial cell.Basic Res. Cardiol. 87:317–332, 1992.
Zeiher, A. M., H. Drexler, H. Wollschlager, and H. Just. Modulation of coronary vasomotor tone. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis.Circulation 83:391–401, 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wong, A.Y.K., Klassen, G.A. A model of electrical activity and cytosolic calcium dynamics in vascular endothelial cells in response to fluid shear stress. Ann Biomed Eng 23, 822–832 (1995). https://doi.org/10.1007/BF02584481
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02584481