Abstract
Background
Abnormal areas in normal-appearing falt colonic mucosa (field defects) may predispose individuals to colon cancer. Markers of field defects would indicate cancer risk.
Methods
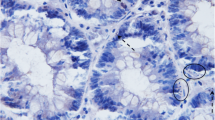
We evaluated apoptosis capability, dedifferentiation, frequency of simple aberrant crypts, aberrant crypt foci, microadenomas, and total nicotinamide adenine dinucleotide levels at locations with normal-appearing flat mucosa obtained from colon resections.
Results
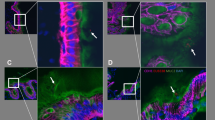
Among goblet cells from colonic mucosa samples of individuals without colonic neoplasia, there was a high mean deoxycholate-induced apoptotic index (AI) of 59.1% and highDolichos biflorus agglutinin (DBA) lectin reactivity (differntiation) in 85.0% of samples. In contrast, flat mucosa samples from colon cancer patients had a significantly (P<.01) lower average AI of 37.4%, and a significantly (P=.03) lower percentage (40.5%) had high DBA reactivity. For colon cancer patients, AI and DBA reactivity values were patchy within a resection. Nicotinamide adenine dinucleotide levels were highly variable among individuals without neoplasia, and aberrant crypt focia and microadenomas were rare.
Conclusions
AI and aberrant DBA reactivity are promising indicators of colon cancer risk. Our results attest to the importance of obtaining multiple samples to assess colon cancer risk because of the patchy nature of field defects.
Similar content being viewed by others
References
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis.Cell 1990;61:759–67.
Issa J-PJ, Vertino PM, Boehm CD, Newsham IF, Baylin SB. Switch from monoallelic to biallelic humanIGF2 promoter methylation during aging and carcinogenesis.Proc Natl Acad Sci USA 1966;93:11757–62.
Baylin S. Tying it all together: epigenetics, genetics, cell cycle, and cancer.Science 1997;277:1948–9.
Bernstein C, Bernstein H, Payne CM, Garewal H. Field defects in progression to adenocarcinoma of the colon and esophagus.Electronic J Biotechnol (online) 2000;3. Available at: http://www. ejb.org/content/vol3/issue3/full/1/. Accessed December 15, 2000.
Payne CM, Bernstein H, Berntein C, Garewal H. Role of apoptosis in biology and pathology: resistance to apoptosis in colon carcinogenesis.Ultrastruct Pathol 1995;19:221–48.
Garewal H, Bernstein H, Bernstein C, Sampliner R, Payne C. Reduced bile acid-induced apoptosis in “normal” colorectal mucosa: a potential biological marker for cancer risk.Cancer Res 1996;56:1480–3.
Bernsein C, Bernstein H, Garewal H, et al. A bile acid-induced apoptosis assay for colon cancer risk and associated quality control studie.Cancer Res 1999;59:2353–7.
Corbe SW, Clarke AR, Gledhill S, Wyllie AH. P53-dependent and-independent links between DNA-damage, apoptosis and mutation frequency in ES cells.Oncogene 1999;18:1537–44.
Kuo M-L., Shiah S-G, Wang C-J, Chuang S-E. Suppression of apoptosis by Bcl-2 to enhance benzene metabolites-induced oxidative DNA damage and mutagenesis: a possible mechanism of carcinogenesis.Mol Pharmacol 1999;55:894–901.
Williams GT, Critchlow MR, Hedge VL, O’Hare KB. Molecular failure of apoptosis: inappropriate cell survival and mutagenesis?Toxicol Lett 1998;102–103:485–9.
Heerdt BG, Houston MA, Augelicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines.Cancer Res 1994;54:3288–94.
Hague A, Manning AM, Hanlon KA, Huschtscha LI, Hart D, Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possille role of dietary fibre in the prevention of large-bowel cancer.Int J Cancer 1993;55:498–505.
Stewart MS, Davis RL, Walsh LP, Pence BC. Induction of differentiation and apoptosis by sodium selenite in human colonic carcinoma cells (HT29).Cancer Lett 1997;117:35–40.
Chang W-CL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation.J Nutr 1997:128:491–7.
Jackson AL, Loeb LA. The mutation rate and cancer.Genetics 1998;148:1483–90.
Payne CM, Crowley C, Washo-Stultz D, et al. The stress-response proteirs poly(ADP-ribose) polymerase and NF-αB protect against bile salt-induced apoptosis.Cell Death Differ 1998;5:623–36.
Payne CM, Bernstein C, Bernstein H. Apoptosis overview emphasizing the role of oxidative stress. DNA damage and signal trans-duction pathways.Leuk Lymphoma 1995:19:43–93.
Boland CR, Martin MA, Goldstein IJ. Lectin reactivities as intermediate biomarkers in premalignant colorectal epithelium.J Cell Biochem Suppl 1992;16G:103–9.
Bresalier RS, Boland CR, Kim YS. Regional differences in normal and cancer-associated glycoconjugates of the human colon.J Natl Cancer Inst 1985;75:249–60.
McLellan EA, Medline A, Bird RP. Sequential analyses of the growth and morphological characteristics of aberrant crypt foci: putative preneoplastic lesions.Cancer Res 1991;51:5270–4.
Pretlow TP, O’Riordan MA, Pretlow TG, Stellato TA. Aberrant crypts in human colonic mucosa: putative preneoplastic lesions.J Cell Biochem Suppl 1992;16G:55–62.
Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer.N Engl J Med 1998;339:1277–84.
Jacobson EL, Jacobson KK. Tissue NAD as a biochemical measure of niacin status in humans.Methods Enzymol 1997;280:221–30.
Jacobson EL, Shieh WM, Huang AC. Mapping the role of NAD metabolism in cancer prevention and treatment.Mol Cell Biochem 1999;193:69–74.
Cherbonnel-Lasserre C, Gauny S, Kronenberg A. Suppression of apoptosis by Bcl-2 or Bcl-xL promotes susceptibility to mutagenesis.Oncogene 1996;13:1489–97.
Liu Y, Naumovski L, Hanawalt P. Nucleotide excision repair capacity is attenuated in human promyelocytic HL60 cells that overexpress Bcl2.Cancer Res 1997;57:1650–3.
Wargovich MJ, Medline A, Bruce WR. Early histopathologic events to evolution of colon cancer in C57BL/6 and CF1 mice treated with 1.2-dimethylhydrazine.J Natl Cancer Inst 1983;71:125–31.
Davies MJ, Bowey EA, Adlercreutz H, Rowland IR, Rumsby PC. Effects of soy or rye supplementation of high-fat diets on colon tumour development in azoxymethane-treated rats.Carcinogenesis 1999;20:927–31.
Roncucci L, Medline A, Bruce WR. Classification of aberrant crypt foci and microadenomas in human colon.Cancer Epidemiol Biomarkers Prev 1991;1:57–60.
Jacobson EL. Niacin deficiency and cancer in women.J Am Coll Nutr 1993;12:412–6.
Jarrett P, Duffill M, Oakley A, Smith A. Pellagra, azathioprine and inflammatory bowel disease.Clin Exp Dermatol 1997;22:44–5.
Cristovao L, Lechner MC, Fidalgo P, Leitao CN, Mira FC, Rueff J. Absence of stimulation of poly(ADP-ribose) polymerase activity in patients predisposed to colon cancer.Br J Cancer 1998;77:1628–32.
Boland CR, Montgomery CK, Kim YS. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation.Proc Natl Acad Sci U S A 1982;79:2051–5.
Jass JR, Roberton AM. Colorectal mucin histochemistry in health and disease: a critical review.Pathol Int 1994;44:487–504.
Raedler A, Raedler E. The use of lections to study normal differentiation and malignant transformation.J Cancer Res Clin Oncol 1985;109:245–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernstein, H., Holubec, H., Warneke, J.A. et al. Patchy field defects of apoptosis resistance and dedifferentiation in flat mucosa of colon resections from colon cancer patients. Annals of Surgical Oncology 9, 505–517 (2002). https://doi.org/10.1007/BF02557276
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02557276