Abstract
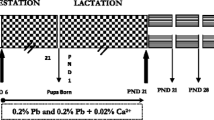
Effect of lead exposure and iron-deficiency on polyamine levels in neuronal and glial cells of cerebellum and hippocampus was investigated in weaned rats. Lactating dams with one day old litters were given 0.2% (w/v) lead acetate in drinking water from postnatal day one to twenty one and maintained on an iron-deficient diet. There was an overall reduction of putrescine, spermidine and spermine in neuronal and glial cells of cerebellum and hippocampus consequent to lead exposure and iron-deficiency alone. Lead exposure and iron-deficiency together did not potentiate the polyamine levels in neuronal and glial cells of cerebellum and hippocampus uniformly. However, the enhanced lowering of putrescine in the hippocampal glia, spermidine in cerebellar neuronal and spermine in both neuronal and glial cells of cerebellum during the critical stage of brain development may result in stunted neuronal growth and sprouting in lead exposed and iron-deficient animals. The behavioral alterations as observed in the present study may be due to impaired neuronal development resulting from a depressed polyamine pathway and which could be attributed to cognitive deficits in growing children.
Similar content being viewed by others
References
Kater, S. B., Mattsor, M. P., Cohan, C., and Connor, J. 1988. Calcium regulation of the neuronal growth cone. Trends Neurosci. 11:315–321.
Legare, M. E., Castiglioni, A. J., Rowles, T. K., Calvin, J. A., Snydr-Armstead, C., and Tiffany Castiglioni, E. 1993. Morphological alterations of neurons and astrocytes in guinea pigs exposed to low levels of inorganic lead. Neurotoxicol. 14:77–80.
Goldstein, G. W. 1990. Lead poisoning and brain cell function. Env. Hlth. Perspectives 89:91–94.
Pounds, J. G., and Cory-slechta, D. A. 1993. New dimensions of lead neurotoxicity: redefining mechanisms and effects. Neurotoxicol. 14:4–6.
Yehuda, S., and Youdim, M. B. H. 1989. Brain iron: a lesson from animal models. Am. J. Clin. Nutr. 56(Suppl.):618–625.
Shaw, G. G., and Pateman, J. J. 1973. The regional distribution of polyamines, spermindine and spermine in brain. J. Neurochem. 20:1225–1230.
Shaw, G. G. 1979. The polyamines in the central nervous system. Biochem. Pharmacol. 28:1–6.
Tabor, H., and Tabor, C. W. 1984. Polyamines. Ann. Rev. Biochem. 53:749–790.
Scott, R. H., Sutton, K. G., and Dolphin, A. C. 1993. Interactions of polyamines with neuronal ion channels. Trends Neurosci. 16: 153–160.
Anand, R., Gore, M. G., and Kerkut, G. A. 1976. The effect of spermine and spermidine on the hydrolysis of acetylthiocholine in the presence of rat caudate nucleus homogenate on acetyl cholinesterase fromElectrophorous electricus. J. Neurochem. 27:381–385.
Iqbal, Z., and Koenig, H. 1985. Polyamines appear to be second messengers in the mediating Ca2+ fluxes and neurotransmitter release in potassium depolarized synaptosomes. Biochem. Biophys. Res. Commun. 133:563–573.
Lopatkin, A. N., Makhina, E. N., and Nichols, C. G. 1994. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372:366–369.
Bell, J. M., and Slotkin, T. A. 1986. Polyamines as intermediates in developmental neurotoxic events. Neurotoxicol. 7:147–160.
Gilad, G. M., Dornay, M., and Gilad, V. H., 1989. Polyamines induce precocious development in rats. Possible interaction with growth factors. Int. J. Dev. Neurosci. 7:641–653.
Zawia, N. H., Evers, L. B. and Harry, G. J. 1994. Developmental profiles of ornithine decarboxylase activity in the hippocampus, neocortex and cerebellum: modulation following lead exposure. Int. J. Dev. Neuroscience 12:25–30.
Miller, G. D., Massaro, T. F., and Massaro, J. E. 1990. Interaction between lead and essential elements: a review. Neurotoxicol. 11: 99–120.
Ragan, H. A. 1977. Effects of iron-deficiency on the absorption of lead and cadmium in rats. J. Lab. Clin. Med. 90:700–706.
Crosby, W. H., and Houchin, D. N. 1957. Preparing a standard solution of cyanmethhaemoglobin. Blood 12:1132–1136.
Vorhees, C. V., Butcher, R. E., Brunner, R. L., and Sobokta, T. J. 1979. A developmental test battery for neurobehavioral toxicity in rats: a preliminary analysis using monosodium glutamate, calcium carageenan and hydroxyurea. Toxicol. Appl. Pharmacol. 50: 267–282.
Kuhn, W. L., and Van Mannen 1961. Central nervous system effects of thalidomide. J. Pharmacol. Exp. Ther. 134:60–68.
Glowinski, J., and Iversen, L. L. 1966. Regional studies of the catecholamines in the rat brain. J. Neurochemistry 13:655–669.
Hamberger, A., Eriksson, O., and Norby, K. 1971. Cell size distribution in brain suspensions and in fractions enriched with neuronal and glial cells. Exp. Cell Res. 67:380–388.
Seiler, N., and Lamberty, V. 1975. Interaction between polyamines and nucleic acids: changes of polyamines and nucleic acids in developing rat brain. J. Neurochem. 24:1–16.
Husain, R., Malaviya, M., Seth, P. K., and Husain, R. 1994. Effect of deltamethrin on regional brain polyamines and behavior in young rats. Pharmacol. Toxicol. 74(4–5):211–215.
Zar, H. J. 1984. Multifactorial analysis of variance. Pages 244–251, in Zar, H. J. (ed.), Biostatistical Analysis, Prentice Hall, New Jersey.
Cookman, G. R., King, W., and Regan, C. W. 1987. Chronic low level lead exposure impairs embryonic to adult conversion of neural cell adhesion molecule. J. Neurochem. 49:399–403.
Ferchmin, P. A., and Eteronic, V. A. 1987. Role of polyamines in experence dependent brain plasticity. Pharmacol. Biochem. Behavior 26:341–349.
Husain, R., Malaviya, M., Seth, P. K., and Husain, R. 1992. Differential responses of regional brain polyamines following in vitro exposure to synthetic pyrethroid insecticides. A preliminary report. Bull. Environ. Contam. Toxicol. 49:402–409.
Youdim, M. B. H. 1990. Developmental, neuropharmacological and biochemical aspects of iron-deficiency. Pages 83–133,in Dobbing, J. (ed.), Brain, Behavior and Iron-deficiency, Springer, New York.
Youdim, M. B. H., Ben-Shachar, D., and Riederer, P. 1989. Is Parkinson's disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta Neurol. Scand. 26:47–54.
Youdim, M. B. H., Ben-Shachar, D., and Yehuda, S. 1989. Putative biological mechanisms of the effect of iron-deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 50:607–616.
Shukla, A., Agarwal, K. N., and Shukla, G. S. 1989. Effect of latent iron-deficiency on metal levels of rat brain regions. Biol. Trace Elem. Res. 22:141–152.
Williams, R. B., and Mills, C. F. (1970). The experimental production of zinc deficiency in rats. Brt. J. Nutr. 24:989–1003.
Hubbel, R. B., Mendel, K. B., and Wakeman, A. J. 1937. A new salt mixture for use in experimental diets. J. Nutr. 14:273–285.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adhami, V.M., Husain, R., Husain, R. et al. Influence of iron deficiency and lead treatment on behavior and cerebellar and hippocampal polyamine levels in neonatal rats. Neurochem Res 21, 915–922 (1996). https://doi.org/10.1007/BF02532341
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02532341