Abstract
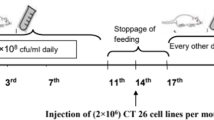
We examined whether the Streptococcal preparation OK-432, an immunopotentiating agent, increases immunocompetence of the gut-associated lymphoid system (GALS), inhibits gastrointestinal carcinogenesis, and has an anti-tumor effect.14C-labelled OK-432 was orally and intraperitoneally administered to rats, and the distribution of the agent in various organs then serially evaluated. The concentration of OK-432 in Peyer's patches and mesenteric lymph nodes was higher after oral administration than after intraperitoneal administration, and showed a biphasic pattern peaking at 30 minutes and 5 hours following administration, in the Peyer's patches. With regard to immunocompetence, PHA- and PWM-stimulated blastogenesis of lymphocytes derived from the mesenteric lymph nodes and peripheral blood enhanced, and the helper/suppressor T-cell ratio was elevated after the oral administration of OK-432. Moreover, chemotactic activity of peritoneal macrophages was also increased. ENNG-induced gastrointestinal carcinogenesis was observed in 60 per cent of the rats orally administered OK-432 as compared with 88 per cent of the controls. The 13-month survival rate of the rats with gastrointestinal cancer was 50 per cent in those administered OK-432 as compared with 25 per cent in those administered OK-432 as compared with 25 per cent in the controls. When administered orally, the agent prevented reduction in immunocompetence in the course of carcinogenesis, suppressed carcinogenesis, and prolonged the survival of animals with cancer without any of the side effects associated with injection. The oral administration of OK-432 is thus considered to be an effective non-specific immunotherapy against gastrointestinal malignancies.
Similar content being viewed by others
References
Kurokawa Y. Experiments on lymph node metastasis by intralymphatic inoculation of rat ascites tumor cells, with special reference to lodgement, possage and growth, of tumor cells in lymph nodes. Gann 1970; 51: 461–471.
Nairn RC, Nind AP, Guli EP. Immunological reactivity in patients with carcinoma of colon. Brit Med J 1971; 18: 706–709.
Nind AP, Nairn RC. Lymphocyte energy in patients with carcinoma. Brit J Cancer 1973; 28: 108–117.
Takeshita M. Non specific cell-mediated immunity in gastric cancer patients—with special reference to immunoreactivity of regional lymph node and preoperative immunotherapy. J Jpn Surg Soc 1983; 84: 679–691.
Tsuchitani T, Kodama H, Tobe T, Nio Y, Kan N, Inamoto T. Oral administration of streptococcal preparation “OK-432”—The 4th report: Augumentation mentation of inin vitro anti tumor immune response of mice with transplanted cecal tumor by oral administration of OK-432—. Jpn Soc Cancer Ther 1984; 19: 2179–2187.
Nakajima Y, Akimoto M, Kimura M, Iwasaki H, Matano S, Hirakawa H, Mori S. Metastatic behavior of footpad-inoculated tumor in mice and the impact of OK-432 per as administration. Jpn J Cancer Chemother 1987; 14: 1229–1233.
Uchida A, Hoshino T. Clinical studies on cell mediated immunity in patients with malignant disease, I Effect of immunotherapy with OK-432 on lymphocyte subpopulations and phytomitogen responsivenessin vitro. Cancer 1980; 45: 476–481.
Oldham RK. Biological response modifier program. J Biol Resp Modif 1982; 1: 82–100.
Uchida A, Hoshino T. Reduction of suppressor cells in cancer patients treated with OK-432 immunotherapy. Intern J Cancer 1980; 26: 401–412.
Nakano H, Ohtani T, Sugawara Y. Studies on absorption, distribution, metabolism and excretion of streptococcal anticancer agent OK-432 in tumor-bearing mice. Proc. 3rd Symp. on Drug Metabolism and Action. Nov. 19–20, 1971 in Fukuoka Japan.
Ogita M. ENNG (N-Ethyl-N'-Nitro-N-Nitrosoguanidine)-induced gastrointestinal carcinogenesis and the suppressive effect of an antimetabolic agent. J Jpn Surg Soc 1979; 81: 1437–1446.
Wanebo HJ, Thaler HT, Hansen JA. Immunologic reactivity in patients with primary operable breast cancer. Cancer 1978; 41: 84–94.
Rittershaus WC, Healey KW, Struzziero CC, Hansen PW. Rapid enumeration of T lymphocytes by a flow cytometric immunofluorescence method. Clinical Chemistry 1982; 28: 1905–1909.
Brideau RJ, Carter PB, McMaster RR, Mason DW, Williams AF. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol 1980; 10: 609–615.
Kumagai K, Itoh K, Hinuma S, Tada M. Pretreatment of plastic petri dishes with fetal calf serum. A sample method for macrophage isolation. J Immunol Methods 1979; 29: 17–25.
Kurashige S, Mitsuhashi S. Macrophage activities in sarcoma 180-bearing mice and EL 4-bearing mice. Gann 1982; 73: 85–90.
Muller-Schoop JW, Good RA. Functional studies of Peyer's patches: Evidence for their participation in intestinal immune response. J Immunol 1975; 114: 1757–1760.
Rothberg RM, Kraft SC, Michalek SM. Systemic immunity after local antigenic stimulation of the lymphoid tissue of the gastrointestinal tract. J Immunol 1973; 115: 1906–1913.
Rothberg RM, Kraft SC. Systemic immunity after local antigenic stimulation of the lymphoid tissue of the gastrointestinal tract. J Immunol 1973; 111: 1906–1913.
Hopkins J, Hall JG. Selective entry of immunoblasts into gut from intestinal lymph. Nature 1976; 259: 308–309.
McWilliams M, Phillips-Quagliata JM, Lam ME. Characteristics of mesenteric lymph node cells homing to gut-associated lymphoid tissue in syngeneix mice. J Immunol 1975; 115: 48–54.
Matsumoto S, Nagai K, Yoshizaki S. Analysis of cytotoxicity induced in thoracic duct lymphocytes by oral administration of a streptococcal preparation, OK-432. JJGS 1986; 19: 2159–2161.
Warshaw AL. Protein uptake by the intestine. Evidence for absorption of intact macromolecules. Gastroenterology 1974; 66: 987–992.
Sugimura T, Fujimura S. Tumor production in glandular stomach of rat by N-Methyl-N'-Nitro-N-Ni-resoguanidine. Nature 1967; 216: 943–948.
Sugimura T, Fujimura S, Kogure K. Production of adenocarcinomas in glandular stomach of experimental animals by N-Methyl-N'-Nitro-N-Nitrosoguanidine. Gann Monograph 1969; 8: 157–162.
Kurihara M, Sumida M, Izumi M. Production of carcinoma in the stomach of experimental dogs by N-Methyl-N'-Nitro-N-Nitrosoguanidine. Igaku no Ayumi 1973; 84: 134–138. (in Japanese)
Giunts JL, Reif AE, Shkler G. Bacillus calmette-Guerine and anti lymphocyte serum in carcinogenesis. Arch Pathol 1974; 98: 237–240.
Johnson S. The effect of thymectomy and of the dose of 3-methyl cholanthrene on the induction and antigenic properties of sarcomas in C57 BL mice. Brit J Cancer 1968; 22: 93–104.
Peissens WF, Heimann R, Legros N, Heuson JC. Effect of bacillus calmetteguerin on mammary tumor formation and cellular immunity in dimethylbenz(a)-anthracene-treated rats. Cancer Res 1971; 31: 1061–1065.
Eisenberg E, Shklar G. Levamisole and hamster pouch carcinogenesis. Oral Surg 1977; 43: 562–571.
Tsunemi S, Fujita Y, Majima S. The effect of levamisole on carcinogenesis in the glandular stomach of rats by ENNG. 1983; 124: 642–646. (in Japanese)
Nio Y, Inamoto T, Hori T, Kan N, Tsuchitani T, Kodama H, Tobe T. The oral administration of OK-432—The 5th report: The effects on lymphoproliferative response and natural killer cell activity in mice with transplanted cecal tumors— Nippon Gann Chiryou Gakkai Zasshi (J Jpn Soc Cancer Ther) 1985; 20: 597–608. (in Japanese)
Minaguchi S. A study on the effects of various immunomodulators on ENNG carcinogenesis of rats. Kitakanto Med J 1988; 38: 41–53.
Nio Y, Ohgaki K, Inamoto T. Oral administration of streptococcal preparation “OK-432 (Picibanil)”–the second report: Experimental studies on its absorption and the responses of the gut associated lymphoid tissue. Nippon Gann Chiryou Gakkai Zasshi (J Jpn Soc Cancer Ther) 1984; 19: 71–79. (in Japanese)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takeshita, M., Makita, F., Takeyoshi, I. et al. Anti-tumor effects of the oral administration of the streptococcal preparation OK-432 (PICIBANIL) — the inhibition of carcinogenesis and growth in rats with ENNG-induced gastrointestinal tumors. The Japanese Journal of Surgery 20, 316–326 (1990). https://doi.org/10.1007/BF02470667
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02470667