Summary
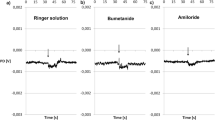
The transepithelial efflux of sodium, from the inner to the outer surface, was measured across the isolated toad skin, mostly after abolition of the electrochemical gradient. The effects on this efflux of several agents and manipulations were studied in order to make a distinction between the paracellular component and a hypothetical transcellular one. Amiloride decreased the transepithelial efflux, while ouabain and cyanide increased it. From the known mode of action of those agents, it was inferred that part of the efflux occurred across the cell. Removal of sodium from the external solution interfered apparently with both components of the transepithelial efflux, while the action of external hypertonicity seemed to be restricted to the paracellular shunt pathway. Access of sodium from the internal solution to the active transport pool is thus suggested, with consequent increase in metabolic cost of transport. Yet, compared with the net influx, the amounts involved are very small; consequently, they escape detection by oxygen consumption measurements.
Similar content being viewed by others
References
Beauwens, R., Al-Awqati, Q. 1976. Further studies on the coupling between sodium transport and respiration in toad urinary bladder.Am. J. Physiol. 231:222
Bentley, P.J. 1968. Amiloride: A Potent inhibitor of sodium transport across the toad bladder.J. Physiol. (London) 195:317
Biber, T.U.L., Mullen, T.L. 1976. Saturation kinetics of sodium efflux across isolated frog skin.Am. J. Physiol. 231:995
Biber, T.U.L., Mullen, T.L. 1977. Effect of inhibitors on transepithelial efflux of Na and nonelectrolytes in frog skin.Am. J. Physiol. 232:C67
Bindslev, N., Tormey, J., Pietras, R.J., Wright, E.M. 1974. Electrically and osmotically induced changes in permeability and structure of toad urinary bladder.Biochim. Biophys. Acta 332:286
Canessa, M., Labarca, P., Leaf, A. 1976. Metabolic evidence that serosal sodium does not recycle through the active transepithelial transport pathway of toad bladder.J. Membrane Biol. 30:65
Civan, M.M. 1970. Effects of active sodium transport on current-voltage relationship of toad bladder.Am. J. Physiol. 219:234
Crabbé, J., Fanestil, D.D., Pelletier, M., Porter, G.A. 1974. Effect of ouabain on sodium transport across hormones-stimulated toad bladder and skin.Pfluegers Arch. 347:275
Davies, H.E.F., Martin, D.G., Sharp, G.W.G. 1968. Differences in the physiological characteristics of bladders of toads from different geographical sources.Biochim. Biophys. Acta. 150:315
Di Bona, D.R., Civan, M.M. 1973. Pathways for movement of ions and water across urinary bladder. I. Anatomic site of transepithelial shunt pathways.J. Membrane Biol. 12:101
Ehrlich, E.N., Crabbé, J. 1968. The mechanism of action of amipramizide.Pfluegers Arch. 302:79
Erlij, D., Martinez-Palomo, A. 1973. Opening of tight junctions in urinary bladders by hypertonic solutions.Fed. Proc. 32:218 (Abstr.)
Fanestil, D.D., Porter, G.A., Edelman, I.S. 1967. Aldosterone stimulation of sodium transport.Biochim. Biophys. Acta 13674
Finn, A.L. 1976. Changing concepts of transepithelial sodium transport.Physiol. Rev. 56:453
Frazier, H.S., Dempsey, E.F., Leaf, A. 1962. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin.J. Gen. Physiol. 45:529
Handler, J.S., Preston, A.S., Orloff, J. 1972. Effect of ADH, aldosterone, ouabain and amiloride on toad bladder epithelial cells.Am. J. Physiol. 222:1071
Helman, S.I., Fisher, R.S. 1977. Microelectrode studies of the active Na transport pathway of frog skin.J. Gen. Physiol. 69:571
Herrera, F.C. 1966. Action of ouabain on sodium transport in the toad urinary bladder.Am. J. Physiol. 210:980
Hviid Larsen, E. 1973. Effect of amiloride, cyanide and ouabain on the active transport pathway in toad skin.In: Transport mechanisms in epithelia. H.H. Ussing and N.A. Thorn, editors. p. 131. Munksgaard, Copenhagen
Kedem, O., Essig, A. 1965. Isotope flows and flux ratios in biological membranes.J. Gen. Physiol. 48:1947
Macknight, A.D.C., Civan, M.M., Leaf, A. 1975. The sodium transport pool in toad urinary bladder epithelial cells.J. Membrane Biol. 20:365
Mandel, L.J., Curran, P.F. 1972. Response of the frog skin to steady-state voltage clamping.J. Gen. Physiol. 59:503
Morel, F. Leblanc, G. 1973. Kinetics of sodium and lithium accumulation in isolated frog skin epithelium.In: Transport mechanisms in epithelia. H.H. Ussing and N.A. Thorn, editors. p. 73. Munksgaard, Cophenhagen
Nagel, W. 1976. The intracellular electrical potential profile of the frog skin epithelium.Pfluegers Arch. 365:135
Noé, G., Michotte, A., Crabbé, J. 1977. Aerobic metabolism of frog skin and its isolated layer as a function of their sodium transporting activity.Biochim. Biophys. Acta 461:231
Reuss, L., Finn, A.L. 1975. Effects of changes in the composition of the mucosal solution on the electrical properties of the toad urinary bladder epithelium.J. Membrane Biol. 20:191
Rick, R., Dörge, A., Bauer, R., Thurau, K. 1976. Syncitial Na transport compartment in epithelia of frog skin and toad urinary bladder.Pfluegers Arch. 365:R11 (Abstr.)
Saito, T., Lief, P.D., Essig, A. 1974. Conductance of active and passive pathways in the toad bladder.Am. J. Physiol. 226:1265
Sudou, K., Hoshi, T. 1977. Mode of action of amiloride in toad urinary bladder.: An electrophysical study of the drug action on sodium permeability of the mucosal border.J. Membrane Biol. 32:115
Ussing, H.H. 1966. Anomalous transport of electrolytes and sucrose through the isolated frog skin induced by hyerptonicity of the outside bathing solution.Ann. N. Y. Acad. Sci. 137:543
Ussing, H.H. 1969. The interpretation of tracer fluxes in terms of membrane structure.Q. Rev. Biophys. 4:365
Ussing, H.H., Windhager, E.E. 1964. Nature of shunt path and active sodium transport path through frog skin epithelium.Acta Physiol. Scand. 61:484
Ussing, H.H., Zerahn, K. 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin.Acta Physiol. Scand. 23:110
Voûte, C.L., Hänni, S. 1973. Relation between structure and function in frog skin.In: Transport Mechanisms in Epithelia. H.H. Ussing and N.A. Thorn, editors. p. 98. Munksgaard, Cophenhagen
Wade, J.B., Revel, J.P., Di Scala, V.A. 1973. Effect of osmotic gradients on intracellular junctions of the toad bladder.Am. J. Physiol. 224:407
Walser, M. 1969. Reversible stimulation of sodium transport in the toad bladder by stretch.J. Clin. Invest. 48:1714
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beauwens, R., Noé, G. & Crabbé, J. Evidence for a transcellular component to the transepithelial sodium efflux in toad skin. J. Membrain Biol. 40 (Suppl 1), 29–43 (1978). https://doi.org/10.1007/BF02025997
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02025997