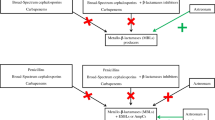
Abstract
Haemophilus influenzae has become increasingly resistant to beta-lactam antibiotics. Three major mechanisms, both enzymatic and non-enzymatic, are involved. Enzymatic resistance is mainly due to production of a TEM-1 plasmid-mediated beta-lactamase, and in some cases to a new enzyme ROB-1. Of the non-enzymatic mechanisms, decreased permeability due to alteration of outer membrane proteins seems to be rare in comparison to decreased affinity of penicillin-binding proteins for beta-lactam antibiotics. Enzymatic resistance is present in about 10–20% of clinical isolates, while non-enzymatic resistance is present only in 2–4%.
Similar content being viewed by others
References
Long, S. S., Teter, M. I., Gilligan, P. H. Biotype ofHaemophilus influenzae: correlation with virulence and ampicillin resistance. Journal of Infectious Diseases 1983, 147: 800–806.
Nikaido, H., Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiological Review 1985, 49: 1–32.
Collatz, E., Gutmann, L. Bacterial porins as mediators of antibiotic susceptibility. In: Peterson, P. K., Verhoef, J., (ed.): The antimicrobial agents annual. Volume 2. Elsevier, Amsterdam, 1987, p. 442–446.
Coulton, J. W., Mason, P., Dorrance, D. The permeability barrier ofHaemophilus influenzae type b againstβ-lactam antibiotics. Journal of Antimicrobial and Chemotherapy 1983, 12: 435–445.
Vachon, V., Lyew, D. J., Coulton, J. W. Transmembrane permeability channel across the outer membrane ofHaemophilus influenzae type b. Journal of Bacteriology 1985, 162: 918–925.
Burns, J. L., Smith, A. L. A major outer-membrane protein functions as a porin inHaemophilus influenzae. Journal of General Microbiology 1987, 133: 1273–1277.
Parr, T. R., Bryan, L. E. Mechanism of resistance of an ampicillin-resistantβ-lactamase negative clinical isolate ofHaemophilus type b toβ-lactam antibiotics. Antimicrobial Agents and Chemotherapy 1984, 25: 747–753.
Makover, S. D., Wright, R., Telep, E. Penicillin-binding inHaemophilus influenzae. Antimicrobial Agents and Chemotherapy 1981, 19: 584–588.
Mendelman, P. M., Chaffin, D. O., Stull, T. L., Rubens, C. E., Mack, K. D., Smith, A. L. Characterization of a nonβ-lactamase-mediated ampicillin resistance inHaemophilus influenzae. Antimicrobial Agents and Chemotherapy 1984, 26: 235–244.
Serfass, D. A., Mendelman, P. M., Chaffin, D. O., Needham, C. Ampicillin resistance and penicillin-binding proteins ofHaemophilus influenzae. Journal of General Microbiology 1986, 132: 2855–2861.
Schryvers, A. B., Wong, S. S., Bryan, L. E. Antigenic relationships among penicillin-binding proteins 1 from members of the familiesPasteurellaceae andEnterobacteriaceae. Antimicrobial Agents and Chemotherapy 1986, 30: 559–564.
Williams, J. D., Andrew, J. Sensitivity ofHaemophilus influenzae to antibiotics. British Medical Journal 1980, i: 134–137.
Kahn, W., Ross, S., Rodriguez, W., Controni, G., Saz, A. K. Haemophilus influenzae type b resistant to ampicillin. Journal of the American Medical Association 1974, 229: 298–301.
Williams, J. D., Kattan, S., Cavanagh, P. Penicillinase production byHaemophilus influenzae. Lancet 1974, ii: 103.
Williams, J. D., Moosdeen, F. Antibiotic resistance inHaemophilus influenzae: epidemiology, mechanisms and therapeutic possibilities. Reviews of Infectious Diseases 1986, 8: S555-S561.
Doern, G. V., Jorgensen, J. H., Thornsberry, C., Preston, D. A., Redding, S. J., Maher, L. A. National collaborative study of the prevalence of antimicrobial resistance among clinical isolates ofHaemophilus influenzae. Antimicrobial Agents and Chemotherapy 1988, 32: 180–185.
Dabernat, H. Activité du réseau de surveillance nationale permanent des infections àHaemophilus influenzae. Bulletin d'Epidémiologie Hebdomadaire 1986, 1: 2–3.
Medeiros, A. A., Levesque, R., Jacoby, G. A. An animal source of ROB-1β-lactamase ofHaemophilus influenzae type b. Antimicrobial Agents and Chemotherapy 1986, 29: 212–215.
De Graaff, J., Elwell, L. P., Falkow, S. Molecular nature of twoβ-lactamase-specifying plasmids isolated fromHaemophilus influenzae type b. Journal of Bacteriology 1976, 126: 439–446.
Jahn, G., Laufs, R., Koulfers, P. M., Kolenda, H. Molecular nature of twoHaemophilus influenzae R factors containing resistance and multiple integration of drug resistance transposons. Journal of Bacteriology 1979, 138: 584–587.
Mendelman, P. M., Doroshow, C. A., Gandy, S. L., Syriopoulou, V., Wrigen, C. P., Smith, A. L. Plasmid mediated resistance in multiply resistantHaemophilus influenzae type b causing meningitis: molecular characterization of one strain and review of literature. Journal of Infectious Diseases 1984, 150: 30–39.
Mendelman, P. M., Syriopoulou, V. P., Gandy, S. L., Ward, J. I., Smith, A. L. Molecular epidemiology of plasmid mediated ampicillin resistance inHaemophilus influenzae type b: isolates from Alaska. Journal of Infectious Diseases 1985, 151: 1061–1071.
Scheifele, D. W., Fussel, S. J., Roberts, M. C. Characterization of ampicillin-resistantHaemophilus para-influenzae. Antimicrobial Agents and Chemotherapy 1982, 21: 734–739.
Moseley, S. L., Samadpour-Motalebi, M., Falkow, S. Plasmid association and nucleotide sequence relationships of two genes encoding heat-stable enterotoxin production inEscherichia coli H-10407. Journal of Bacteriology 1983, 156: 441–443.
Goldstein, F. W., Boisivon, A., Leclerc, P., Acar, J. F. Sensibilité d'Haemophilus sp. aux antibiotiques. Transfert de résistance aEscherichia coli. Pathologie et Biologie 1977, 25: 323–332.
Joly, B., Delmas, C., Rich, C., Prere, M. F., Livielle, V., Dabernat, H. Un nouveau mécanisme de résistance à l'ampicilline par production deβ-lactamase ROB-1 chez une souche d'Haemophilus influenzae isolée en France. La Presse Médicale 1987, 16: 916–917.
Medeiros, A. A., O'Brien, T. Ampicillin resistantHaemophilus influenzae type b possessing TEM typeβ-lactamase but little permeability barrier to ampicillin. Lancet 1975, i: 716–718.
Laferriere, C., Marks, M. I., Welch, D. F. Effect of inoculum size onHaemophilus influenzae type b susceptibility to new and conventional antibiotics. Antimicrobial Agents and Chemotherapy 1983, 24: 287–289.
Syriopoulou, V. Ph., Scheifele, D. W., Sack, C. M., Smith, A. L. Effect of inoculum size on the susceptibility ofHaemophilus influenzae b toβ-lactam antibiotics. Antimicrobial Agents and Chemotherapy 1979, 16: 510–513.
Sanson-Le-Pors, M. J., Casin, I. Haemophilus et antibiotiques In: Courvalin, P., Goldstein, F., Philippon, A., Sirot, J. (ed.): L'Antibiogramme. M.P.C.-Videom, Paris, 1985, p. 81–86.
Barry, L. A., Jones, R. N., Thornsberry, C. Alpacillin (PC-904): spectrum of activity andβ-lactamase hydrolysis inhibition. Diagnostic Microbiology and Infectious Diseases 1985, 3: 7–17.
Sanders, C. C. Comparative activity of mezlocillin, penicillin, ampicillin, carbenicillin and ticarcillin against gram-positive bacteria. Antimicrobial Agents and Chemotherapy 1981, 20: 843–846.
Campos, J., Garia Tornel, S. Comparative susceptibility of ampicillin and chloramphenicol resistantHaemophilus influenzae to fifteen antibiotics. Journal of Antimicrobial Chemotherapy 1987, 19: 297–301.
Campos, J. M., Gill, C. J., Ahonkhai, V. I. In vitro activity of imipenem against 100 strains of serotype b and nontypableHaemophilus influenzae, including strains resistant to ampicillin, chloramphenicol or both. Journal of Antimicrobial Chemotherapy 1985, 16: 549–544.
Liljequist, B. O., Gezelius, L. In vitro activity of amoxycillin plus clavulanic acid againstHaemophilus influenzae andBranhamella catarrhalis. European Journal of Clinical Microbiology 1986, 5: 615–621.
Wise, R., Andrew, J. M., Bedford, K. A. Clavulanic and CP 45-899: a comparison of their in vitro activity in combination with ampicillin. Journal of Antimicrobial Chemotherapy 1980, 6: 197–201.
Gutmann, L., Kitzis, M. D., Yamabé, S., Acar, J. F. Comparative evaluation of a newβ-lactamase: inhibitor, YTR 830, combined with differentβ-lactam antibiotics against bacteria harboring knownβ-lactamases. Antimicrobial Agents and Chemotherapy 1986, 29: 955–957.
Newsom, S. B. W., Mattews, J. Ampicillin resistance inHaemophilus influenzae. Test method for activity of acylureidopenicillins, cephamycins and new cephalosporins. Journal of Antimicrobial Chemotherapy 1982, 10: 527–532.
Malouin, F., Schryvers, A. B., Bryan, L. E. Cloning and expression of genes responsible for altered penicillin-binding proteins 3a and 3b inHaemophilus influenzae. Antimicrobial Agents and Chemotherapy 1987, 31: 286–291.
Chen, H. Y., Williams, J. D. Temocillin compared to ampicillin againstHaemophilus influenzae and with other penicillins against intestinal aerobic gram-negative rods. Journal of Antimicrobial Chemotherapy 1982, 10: 279–287.
Tomasz, A. The mechanism of the irreversible antimicrobial effects of penicillins. Annual Review of Microbiology 1979, 33: 113–137.
Bergeron, M., Lavoie, G. Tolerance ofHaemophilus influenzae toβ-lactam antibiotics. Antimicrobial Agents and Chemotherapy 1985, 28: 320–325.
Liu, H., Tomasz, A. Penicillin tolerance in multipe drug resistant natural isolates ofStreptococcus pneumoniae. Journal of Infectious Diseases 1985, 152: 365–372.
Mandelman, P., Chaffin, D. O., Clausen, C., Stall, T. L., Needham, C., Williams, J. D., Smith, A. Failure to detect ampicillin-resistant, non-β-lactamase producingHaemophilus influenzae by standard disk susceptibility testing. Antimicrobial Agents and Chemotherapy 1986, 30: 274–280.
Philpott Howard, J., Seymour, A., Williams, J. D. Accuracy of methods used for susceptibility testing ofHaemophilus influenzae in United Kingdom laboratories. Journal of Clinical Pathology 1983, 36: 1105–1110.
Zimmerman, W., Rosselet, A. Function of the outer membrane ofEscherichia coli as a permeability barrier toβ-lactam antibiotics. Antimicrobial Agents and Chemotherapy 1977, 12: 368–372.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gutmann, L., Williamson, R., Collatz, E. et al. Mechanisms of beta-lactam resistance inHaemophilus influenzae . Eur. J. Clin. Microbiol. Infect. Dis. 7, 610–615 (1988). https://doi.org/10.1007/BF01964237
Issue Date:
DOI: https://doi.org/10.1007/BF01964237