Abstract
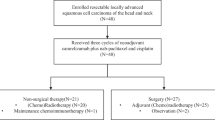
We carried out a pilot nonrandomized phase II study to compare the neo-adjuvant chemotherapic regimen with cisplatin, 5-FU and vinorelbine with the same combination plus s.c. IL 2 in advanced head and neck squamous cell carcinoma (HNSCC). The primary goals of the trial were to evaluate the feasibility and response rates of the two regimens. The study design consisted of a patient's assignment to either of the two following arms: Arm A: Cisplatin 80 mg/m2 i.v. on day 1; 5-FU 600 mg/m2 i.v. on days 2–5; and vinorelbine 20 mg/m2 i.v. on days 2 and 8, Arm B: the same chemotherapic regimen plus recombinant IL 2 (Proleukin, Eurocetus) 9 MIU s.c. daily from day 9 to 13 and from day 16 to 20 for every cycle. From March 1993 to November 1993 twenty three patients with Stage III–IV HNSCC were enrolled in the study. Patients could be evaluated for response to treatment if they had received at least 2 complete cycles of therapy. The overall response rate (ORR) was 63% in Arm A and 100% in Arm B. The differences for ORR and CR rates were statistically significant in favor of Arm B. The analysis for each of the three drugs included in the chemotherapy schedule shows that both the actually received average dose-intensity and the actually delivered average cumulative doses/patient were higher for Arm B (chemo- plus IL 2 therapy) (approximately 80% of programmed dose-intensity) than for Arm A (approximately 70% of programmed dose-intensity). Both the actually received average dose-intensity and the actually delivered average cumulative doses/patient for IL 2 were more than 80%. In both arms the most frequent side effects were myelosuppression, phlebitis and electrolyte disturbances. There were 2 toxic deaths, 1 in Arm A and 1 in Arm B, both for hematologic toxicity. Our “pilot” study suggests that the combination of cisplatin, 5-FU, vinorelbine plus IL 2 is a highly active, but rather toxic, neo-adjuvant treatment in advanced HNSCC with very high ORR and CR rates.
Similar content being viewed by others
References
Million RR, Cassisi NJ. Management of head and neck cancer. JB Lippincott company (Philadelphia) 1994.
Kish JA, Weaver A, Jacobs J. Cisplatin and 5-fluorouracil infusion in patients with recurrent and disseminated epidermoid cancer of the head and neck. Cancer 1984; 53: 1819–24.
Vogl SE, Schoenfeld DA, Kaplan BH. A randomized prospective comparison of methotrexate with a combination of methotrexate, bleomycin and cisplatin in the head and neck cancer. Cancer 1985; 56: 432–42.
Bosl GJ, Strong E, Harrison L, Pfister DG. Chemotherapy and the management of the locally advanced squamous cell carcinoma of the head and neck: Role in larynx preservation: Important Advances in Oncology. DeVita VT jr, Hellman S, Rosenberg SA, eds. J.B. Lippincott company (Philadelphia) 1991; 191–203.
Al-Sarraf M. Head and neck cancer: Chemotherapy concepts. Semin Oncol 1988; 15: 70–85.
Gebbia V, Zerillo G, Gebbia N. Chemotherapy in head and neck cancer (I): Management of recurrent or metastatic disease. J Chemother 1992; 4: 244–59.
Choksi AJ, Dimery IW, Hong WK. Adjuvant chemotherapy of head and neck cancer; the past, the present, the future. Semin Oncol 1988; 15: 45–59.
Depierre A, Lemariè E, Dabouis G. Efficacy of navelbine (NVB) in non-small cell lung cancer (NSCLC). Semin Oncol 1989; 16 (suppl 4): 26–9.
Gebbia V, Testa A, Valenza R. A pilot study of vinorelbine on a weekly schedule in recurrent and or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer 1993; 29A: 1358–9.
Cros S, Wright M, Morimoto C. Experimental antitumor activity of navelbine (5′-Nor-anhydrovinblastine, vinorelbine). Semin Oncol 1989; 16(suppl 4): 15–20.
Leitner SP, Bosl GJ, Strong EW. A pilot study of cisplatinvinblastine as the initial treatment of advanced head and neck cancer. Cancer 1986; 58: 1014–7.
Parkinson DR. Interleukin-2 in cancer therapy. Semin Oncol 1988; 15: 10–26.
Rosenberg SA, Lotze MT, Muul LM. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316: 889–97.
West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of lung cancer. Cancer Immunol Immunother 1987; 24: 76–80.
Figlin RA. Biotherapy with interferon — 1988. Semin Oncol 1988; 6: 3–9.
Spiegel RA. Theα interferons: Clinical overview. Semin Oncol 1987; 14: 1–12.
Jakubowski AA, Casper ES, Gabrilov JL, Templeton MA, Sherwin SN, Oettgen HF. Phase I trial of intramuscularly administered tumor necrosis factor in patients with advanced cancer. J Clin Oncol 1989; 7: 298–303.
Valone FH, Gandara DR, Deisseroth AB. Interleukin-2, cis-platin, and 5-fiuorouracil for patients with non-small cell lung and head/neck carcinomas. J Immunother 1991; 10: 207–13.
Miller AB, Hodgstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14.
Clark J, Dreyfuss A, Busse P. Neo-adjuvant infusion cisplatin, 5-FU and high-dose leucovorin for squamous cell carcinoma of the head and neck (SCCHN): High rates of complete response (CR) and definitive radiotherapy as primary site management: Cancer Treatment: An update. Banzet P, Holland JF Khayat D, Weil M, eds. Springer-Verlag (Paris) 1994; 196–200.
Mantovani G, Contini L, Littera S. Primary (neo-adjuvant) combined modality therapy in the management of locally advanced squamous cell carcinoma of the head and neck: Cancer Treatment: An update. Banzet P, Holland JF, Khayat D, Weil M, eds. Springer-Verlag (Paris) 1994, 201–206.
Fonseca E, Cruz JJ, Garcia J. Induction chemotherapy with cisplatin (P), 5-Fluorouracil (FU) and folinic acid (FA) in locally advanced head and neck cancer: Cancer Treatment: An update. Banzet P, Holland JF, Khayat D, Weil M, eds. Springer-Verlag (Paris) 1994; 211–213.
Kies MS, Haraf DJ, Mittal B. A phase II trial of induction cisplatin, 5-FU, leucovorin and interferonα-2b (PFL-α) followed by concurrent hydroxyurea, 5-FU and radiation for stage IV squamous cell cancers of the head and neck (HNC). Cancer Treatment: An update. Banzet P, Holland JF, Khayat D, Weil M, eds. Springer-Verlag (Paris) 1994: 207–210.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mantovani, G., Bianchi, A., Curreli, L. et al. Neo-adjuvant chemotherapy ± immunotherapy with s.c. IL 2 in advanced squamous cell carcinoma of the head and neck: A pilot study. Biotherapy 8, 91–98 (1994). https://doi.org/10.1007/BF01878492
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01878492