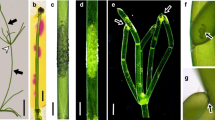
Summary
In the lichenParmelia caperata (L.) Ach. the distribution pattern of membrane-bound Ca2+ is investigated in the symbionts by chlorotetracycline (CTC)-induced fluorescence during the development of propagative structures, the soredia. The results demonstrate that Ca2+ accumulation in the alga and the fungus is associated with this morphogenetic process; particularly, polarized hyphal growth involves a tip-to-base Ca2+ gradient.
CTC fluorescence distribution is coincident with that of cholinesterase (ChE) activity during morphogenesis of soredia. A comparison is suggested with ‘embryonic ChE’ of animal cells, where developmental events are regulated by a cholinergic mechanism that also modulates Ca2+ levels.
Similar content being viewed by others
References
ACHENBACH, F., ACHENBACH, U. & KESSLER, D. (1984) Calcium binding sites in plasmodia ofPhysarum polycephalum as revealed by the pyroantimoniate technique.J. Histochem. Cytochem. 32, 1177–84.
BERRIDGE, M. J. & IRVINE, R. F. (1984) Inositol trisphosphate, a novel second messenger in cellular signal transduction.Nature 312, 315–21.
BRAWLEY, S. H. & ROBINSON, K. R. (1985) Cytochalasin treatment disrupts the endogenous currents associated with cell polarization in fucoid zygotes: studies of the role of F-actin in embryogenesis.J. Cell Biol. 100, 1173–84.
BROWNLEE, C. & WOOD, J. W. (1986) A gradient of cytoplasmic free calcium in growing rhizoid cells ofFucus serratus.Nature 320, 624–6.
CASWELL, A. H. (1979) Methods for measuring intracellular calcium.Int. Rev. Cytol. 56, 145–81.
CHEN, T.-H. & JAFFE, L. F. (1979) Forced clacium entry and polarized growth ofFunaria spores.Planta 144, 101–6.
DETTBARN, W. D. (1962) Acetylcholinesterase activity inNitella.Nature 194, 1175–6.
DREWS, U. (1975) Cholinesterase in embryonic development.Prog. Histochem. Cytochem. 7, 1–52.
EVANS, M. L. (1972) Promotion of cell elongation inAvena coleoptiles by acetylcholine.Plant Physiol. 50, 414–16.
GILROY, S., BLOWERS, D. P. & TREWAVAS, A. J. (1987) Calcium: a regulation system emerges in plant cells.Development 100, 181–4.
GOODWIN, B. C. & PATEROMICHELAKIS, S. (1979) The role of electrical fields, ions, and the cortex in the morphogenesis ofAcetabularia.Planta 145, 427–35.
GOODWIN, B. C., SKELTON, J. L. & KIRK-BELL, S. M. (1983) Control of regeneration and morphogenesis by divalent cations inAcetabularia mediterranea.Planta 157, 1–7.
HOITINK, A. W. & DIJK, G. V. (1965) The influence of neurohumoral transmitter substances on protoplasmic streaming in the MyxomycetePhysarella oblonga.J. Cell Physiol. 67, 133–40.
HOSHINO, T. (1983) Effects of acetylcholine on the growth of theVigna seedlings.Plant & Cell Physiol. 24, 551–6.
JAFFE, M. J. (1970) Evidence for the regulation of phytochrome-mediated processes in bean roots by the neurohumor acetylcholine.Plant Physiol. 46, 768–77.
JAFFE, M. J. (1972) Acetylcholine as a native metabolic regulator of phytochrome-mediated processes in bean roots. InRecent Advances in Phytochemistry. Vol. 5, (edited by RUNECKLES, V. C. & TSO, T. C.) pp. 81–104. New York, Academic Press.
JAFFE, L. A., WEISENSEEL, M. H. & JAFFE, L. F. (1975) Calcium accumulation within the growing tips of pollen tubes.J. Cell Biol. 67, 488–92.
KAUPPI, M. (1984) Fluorescence microscopy for the examination of different tissues in lichens and pollution effects on the algal cell layer. InAbstracts of the VIIth International Congress of Histochemistry and Cytochemistry (edited by PANULA, P., PÄIVÄRINTA, H. & SOINILA, S.) p. 187. Helsinki.
KEITH, C. H., RATAN, R., MAXFIELD, F. R., BAJER, A. & SHELANSKI, M. L. (1985) Local cytoplasmic calcium gradients in living mitotic cells.Nature 316, 848–50.
KROPF, D. L., LUPA, M. D. A., CALDWELL, J. H. & HAROLD, F. M. (1983) Cell polarity: endogenous ion currents precede and predict branching in the water moldAchlya.Science 220, 1385–7.
KROPF, D. L., CALDWELL, J. H., GOW, N. A. R. & HAROLD, F. M. (1984) Transcellular ion currents in the water moldAchlya. Amino acid proton symport as a mechanism of current entry.J. Cell Biol. 99, 486–96.
LALLEMANT, R. (1972) Etude de la formation des sorédies chez le DiscolichenBuellia canescens (Dicks.) D. Notrs.Bull. Soc. Bot. Fr. 119, 463–76.
LEES, G. L. & THOMPSON, J. E. (1975) The effects of germination on the subcellular distribution of cholinesterase in cotyledons ofPhaseolus vulgaris.Physiol. Plant. 34, 230–7.
MCNALLY, J. G., COWAN, J. D. & SWIFT, H. (1983) The effects of the ionophore A23187 on pattern formation in the algaMicrasterias.Dev. Biol. 97, 137–45.
MEINDL, U. (1982) Local accumulation of membrane-associated calcium according to cell pattern formation inMicrasterias denticulata, visualized by chlorotetracycline fluorescence.Protoplasma 110, 143–6.
MIURA, G. A. & SHIH, T.-M. (1984) Cholinergic constituents in plants: characterization and distribution of acetylcholine and choline.Physiol. Plant. 61, 417–21.
MUKHERJEE, I. (1980) The effect of acetylcholine on hypocotyl elongation in soybean.Plant & Cell Physiol. 21, 1657–60.
NAKAJIMA, H. & HATANO, S. (1962) Acetylcholinesterase in the plasmodium of the myxomycete,Physarum polycephalum.J. Cell. Comp. Physiol. 59, 259–64.
OETTLING, G., SCHMIDT, H. & DREWS, U. (1985) The muscarinic receptor of chick embryo cells: correlation between ligand binding and calcium mobilization.J. Cell Biol. 100, 1073–81.
PICTON, J. M. & STEER, M. W. (1982) A model for the mechanism of tip extension in pollen tubes.J. Theor. Biol. 98, 15–20.
RAINERI, M. & MODENESI, P. (1986) Preliminary evidence for a cholinergic-like system in lichen morphogenesis.Histochem J. 18, 647–57.
REISS, H.-D. & HERTH, W. (1978) Visualization of the Ca2+ gradient in growing pollen tubes ofLilium longiflorum with chlorotetracycline fluorescence.Protoplasma 97, 373–77.
REISS, H.-D. & HERTH, W. (1979) Calcium gradients in tip growing plant cells visualized by chlorotetracycline fluorescence.Planta 146, 615–21.
REISS, H.-D. & NOBILING, R. (1986) Quin-2 fluorescence in lily pollen tubes: distribution of free cytoplasmic calcium.Protoplasma 131, 244–6.
RIOV, J. & JAFFE, M. J. (1973) A cholinesterase from bean roots and its inhibition by plant growth retardants.Experientia 29, 264–5.
ROBINSON, K. R. & JAFFE, L. F. (1975) Polarizing fucoid eggs drive a calcium current through themselves.Science 187, 70–2.
ROBINSON, K. R. & CONE, R. (1980) Polarization of fucoid eggs by a calcium ionophore gradient.Science 207, 77–8.
SCHMIDT, H., OETTLING, G., KAUFENSTEIN, T., HARTUNG, G. & DREWS, U. (1984) Intracellular calcium mobilization on stimulation of the muscarinic cholinergic receptor in chick limb bud cells.Roux's Arch. Dev. Biol. 194, 44–9.
TREWAVAS, A. J., SEXTON, R. & KELLY, P. (1984) Polarity, calcium and abscission: molecular bases for developmental plasticity in plants.J. Embryol. Exp. Morph. 83 Suppl., 179–95.
WEISENSEEL, M. H., NUCCITELLI, R. & JAFFE, L. F. (1975) Large electrical currents traverse growing pollen tubes.J. Cell Biol. 66, 556–67.
WICK, S. M. & HEPLER, P. K. (1980) Localization of Ca2+-containing antimonate precipitates during mitosis.J. Cell Biol. 86, 500–13.
WOLNIAK, S. M., HEPLER, P. K. & JACKSON, W. T. (1980) Detection of the membrane-calcium distribution during mitosis inHaemanthus endosperm with chlorotetracycline.J. Cell Biol. 87, 23–32.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raineri, M., Modenesi, P. Membrane-bound Ca2+ distribution visualized by chlorotetracycline fluorescence during morphogenesis of soredia in a lichen. Histochem J 20, 81–87 (1988). https://doi.org/10.1007/BF01746608
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01746608