Abstract
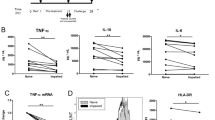
The hypothesis that acute ethanol uptake plus trauma can synergize to increase immunosuppression was tested. We found that, unlike non-alcohol-exposed patients, patients with acute alcohol use prior to trauma have a transient decrease in monocyte tumor necrosis factor α (TNFα) production during the very early postinjury (0–3 days) period. However, TNFα production by these alcoholexposed patients' monocytes (MØ) became hyperelevated late postinjury (>9 days). Consequently, these massively elevated MØ TNFα levels can contribute to posttrauma immunosuppression after acute alcohol use. We also demonstrate that normal monocyte activation with the superantigen,Staphylococcus enterotoxin B (SEB), results in a preferential induction of cellassociated MØ TNFα production, described as characteristic of immunosuppressed trauma patients. Acutein vitro ethanol treatment down-regulated the elevated TNFα production by trauma patients' MØ after either SEB, muramyl-dipeptide (MDP), interferon-γ plus MDP, or lipopolysaccharide (LPS) stimulation. Both SEB- and LPS-induced TNFα mRNA induction was inhibited by acute alcohol treatment in normal MØ, indicating that ethanol can regulate cytokine gene expression. An additional immunosuppressive effect of acute ethanol's stimulation was suggested by its induction of elevated transforming growth factor β production in trauma patients' activated MØ.
Similar content being viewed by others
References
Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E: High IL-6 serum levels and increased production by leukocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokine production. Clin Exp Immunol 77:221–225, 1989
Ewald SJ, Shao H: Ethanol increases apoptotic cell death of thymocytes in vitro. Alcohol Clin Exp Res 17:359–365, 1993
Jerrels TR, Marietta CA, Eckhardt MJ, Majchrowith E, Weight FF: Effects of ethanol administration on parameters of immunocompetency in rats. J Leuk Biol 39:499–510, 1986
Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI: Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 13:267–276, 1991
Meadows GG, Wallendal M, Kosugi A, Wunderlich J, Singer DS: Ethanol induces marked changes in lymphocyte populations and natural killer cell activity in mice. Alcohol Clin Exp Res 16:474–479, 1992
Morland B, Morland H: The interaction of ethanol with human monocyte IgG-Fc receptors, characterized by monoclonal antibodies raised against two distinct receptor subpopulations. Scand J Immunol 29:573–577, 1989
Nelson S, Bagby GJ, Bainton G, Warren WR: The effects of acute and chronic alcoholism on tumor necrosis factor and inflammatory response. J Infect Dis 1989:422–429, 1989
Bermudez LE, Wu M, Martinelli J, Young LS: Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res 10:413–419, 1991
Bermudez LE, Young L: Ethanol augments intracellular survival of Mycobacterium avium complex and impairs macrophage responses to cytokines. J Infect Dis 163:1286–1292, 1991
Szabo G, Verma B, Fogarasi M, Catalano D: Induction and modulation of transforming growth factor β and prostaglandin E2 by ethanol in human monocytes. J Leuk Biol 52:602–611, 1992
Szabo G, Verma B, Catalano D: Selective inhibition of antigen-specific T lymphocyte proliferation by acute ethanol exposure: The role of impaired monocyte antigen presentation capacity and mediator production. J Leuk Biol 54:534–544, 1993
Verma BK, Fogarasi M, Szabo G: Down-regulation of TNFα activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol 13:8–22, 1993
Watson RR: Diagnosis of alcohol abuse by modulation of immune responses.In Diagnosis of Alcohol Abuse, R Watson (ed). Boca Raton, FL, CRC Press, 1989, pp 101–106
Cook RT, Garvey MJ, Booth BM, Goeken JA, Stewart B, Noel M: Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. J Clin Immunol 11:1991
Szabo G: Monocyte-mediated immunosuppression after acute ethanol exposure.In Alcohol, Drugs of Abuse and Immunomodulation. RR Watson (ed). Tarrytown, NY, Pergamon Press, 1993, pp 121–133
Matsuoka M, Tsukamoto H: Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor β: Implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology 11:599–605, 1990
Moscat J, Aracil M, Diez E, Garcia-Barreno P, Municio AM: Effect of ethanol on the arachidonic acid metabolism in mouse peritoneal macrophages. Prostaglandins 34:853–866, 1987
Kawakami M, Meyer AA, DeSerres S, Peterson HD: Effects of acute ethanol ingestion and burn injury on serum immunoglobulin. J Burn Care Rehab 11:395–399, 1990
Kawakami M, Switzer BR, Herzog SR, Meyer AA: Immune suppression after acute ethanol ingestion and thermal injury. J Surg Res 51:210–215, 1991
Miller-Graziano CL, Szabo G, Kodys K, Griffey K: Aberrations in post-trauma monocyte subpopulation: Role in septic shock syndrome. J Trauma 30:S86-S96, 1990
Miller-Graziano C, Szabo G, Griffey K, Metha B, Kodys K, Catalano D: Role of elevated MØ TGFβ production in post-trauma immunosuppression. J Clin Immunol 11:95–102, 1991
Miller-Graziano C, Szabo G, Kodys K: The interactions of immunopathological mediators (TNF, TGFβ, PGE2) in traumatized individuals.In The Immune Sequences of Trauma, Shock, and Sepsis-Mechanisms and Therapeutic Approaches, E Faist (ed). Berlin, Springer-Verlag, 1992, pp 637–650
Faist E, Stork M, Hultner I, Redl H, Ertel W, Waltz A, Schieldberg F: Functional analysis of monocyte activity through synthesis patterns of proinflammatory cytokines and neopterin in patients in surgical intensive care. Surgery 112:562–572, 1992
Damas P, Ledoux D, Nys M, Vrindts Y, DeGrotte D, Franchimont P, Lamy M: Cytokine serum level during severe sepsis in human Il-6 as a marker of severity. Ann Surg 215:356–362, 1991
Ayala A, Perrin MM, Chaudry IH: Defective macrophage antigen presentation following haemorrhage is associated with the loss of MHC class II antigens. Immunology 70:33–39, 1990
Waydhas C, Nast-Kolb D, Jochum M, Trupka A, Lenk S, Fritz H, Schweiberer L: Inflammatory mediators, infections, sepsis, and multiple organ failure after severe trauma. Arch Surg 127:460–467, 1992
Casey LC, Balk RA, Bone RC: Plasma cytokine and endotoxin levels correlate with survival in patients with sepsis syndrome. Ann Intern Med 119:771–778, 1993
Szabo G, Kodys K, Miller-Graziano CL: Elevated monocyte interleukin-6 (IL-6) production in immunosuppressed trauma patients. I. Role of FcγRI cross-linking stimulation. J Clin Immunol 11:326–335, 1991
Jorens P, Van Damme J, De Backer W: Interleukin-8 in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome. Cytokine 4:592–597, 1992
Miller-Graziano CL, Fink M, Wu JY, Szabo G, Kodys K: Mechanisms of altered monocyte prostaglandin E2 production in severely injured patients. Arch Surg 123:293–299, 1988
Ertel W, Morrisson M, Ayala A: Blockade of prostaglandin production increases cachectin synthesis and prevents depression of macrophage functions after hemorrhagic shock. Ann Surg 213:265–271, 1991
Moses T, Ben-Efraim S, Tak C: Serum levels of tumor necrosis factor determine the fatal or nonfatal course of endotoxic shock. Immunol Lett 27:157–162, 1991
Takayama TK, Miller C, Szabo G: Elevated tumor necrosis factor production concomitant to elevated prostaglandin E2 production by trauma patients' monocytes. Arch Surg 125:29–35, 1990
Wherry JC, Pennington JE, Wenzel RP: Tumor necrosis factor and the therapeutic potential of anti-tumor necrosis factor antibodies. Crit Care Med 21(10):S436-S440, 1993
Bodmer M, Fournel MA, Hinshaw LB: Preclinical review of anti-tumor necrosis factor monoclonal antibodies. Crit Care Med 21(10):S441-S446, 1993
Roumen RMH, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GAP, Sauerwein RW, van der Meer JWM, Goris RJA: Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Ann Surg 218(6):769–776, 1993
Heideman M, Kaijser B, Gelin LE: Complement activation and hematologic, hemodynamic, and respiratory reactions early after soft tissue injury. J Trauma 18:696–700, 1978
Morell V: Predicting severity of trauma by admission white blood cell count, serum potassium level, and arterial pH. South Med J 86:658–659, 1993
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159, 1987
Szabo G, Miller-Graziano CL, Wu J, Takayama T, Kodys K: Differential tumor necrosis factor production by human monocyte subsets. J Leuk Biol 47:206–216, 1990
Jacob CO, Lewis GD, HO, McDevitt: MHC ClassII-associated variation in the production of tumor necrosis factor in mice and humans: Relevance to the pathogenesis of autoimmune diseases. Immunol Res 10:156–168, 1991
Szabo G, Kodys K, Miller-Graziano CL: Elevated monocyte IL-6 production in immunosuppressed trauma patients. II. Downregulation by IL-4. J Clin Immunol 11:336–344, 1991
Spengler RN, Spengler ML, Strieter RM, Remick DG, Larrick JW, Kunkel SL: Modulation of tumor necrosis factor-3α gene expression. J Immunol 142:4346–4350, 1989
Dunham DM, Arkins S, Edwards CK, Dantzer R, Kelley KW: Role of Interferon-gamma in counteracting the sup- pressive effects of transforming growth factor-β2 and glucocorticoids on the production of tumor necrosis factor-α. J Leuk Biol 48:473–481, 1990
Tracey KJ: Tumor necrosis factor (cachectin) in the biology of septic shock syndrome. Circ Shock 35:123–128, 1991
Sharma S, Olchowy T, Yang Z: TNFα and IL-1 enhance LPS-mediated bovine endothelial cell injury. J Leuk Biol 51:579–585, 1992
Piquet P, Grau G, Vassali P: TNF and immunopathology. Immunol Res 10:122–140, 1991
Goldman G, Welbourn R, Kobzik L: TNFα mediates acid aspiration-induced systemic organ injury. Ann Surg 212:513–520, 1990
Karkar A, Koshino Y, Cashman S: Passive immunization against TNFα and IL-1β protects from LPS enhancing glomerular injury in nephrotoxic nephritis in rats. Clin Exp Immunol 90:312–318, 1992
Szabo G, Kodys K, Miller-Graziano CL: Dibutyryl-cAMP modulation of receptor expression and antigen presentation capacity in monocyte subpopulations. Int J Immunopharmacol 16(2):151–162, 1994
Fukushima R, Gianotti L, Alexander J: The degree of bacterial translocation is a determinant factor for mortality after burn injury and is improved by prostaglandin analogs. Ann Surg 216:438–444, 1992
Spengler RN, Spengler ML, Lincoln P, Remick DG, Strieter RM, Kunkel SL: Dynamics of dibutyryl cyclic AMP- and prostaglandin E2-mediated suppression of lipopolysaccharide-induced tumor necrosis factor alpha gene expression. Infect Immun 57:2837–2841, 1989
Molloy RG, O'Riordain M, Holzheimer R, Nestor M, Collins K, Mannick JA, Rodrick ML: Mechanism of increased tumor necrosis factor production after thermal injury. J Immunol 151:2142–2149, 1993
Spinas GA, Keller U, Brockhaus M: Release of soluble receptors for TNF in relation to circulating TNF during experimental endotoxemia. J Clin Invest 90:533–536, 1992
Nii A, Sone S, Orino E: Induction of a 26-kDa membrane form TNFα in human alveolar macrophages. J Leuk Biol 53:29–36, 1993
Ayala P, Perrin MM, Wang P: Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity. Involvement of cell-associated tumor necrosis factor and reactive nitrogen. J Immunol 147:4147–4154, 1991
Ware CF, Crowe PD, Grayson MH, Androlewich MJ, Briwning JL: Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol 149:3881–3888, 1992
Kappler J, Kotzin B, Herron L, Gelfand E, Bigler R, Boylston A, Carrel S, Posnatt D, Choi Y, Marrack P: V beta-specific stimulation of human T cells by staphylococcal toxins. Science 244:811, 1989
See RH, Krystal G, Chow AW: Receptors for toxic shock syndrome toxin-1 and staphylococcal enterotoxin A on human blood monocytes. Can J Microbiol 38:937–944, 1992
See RH, Kum WWS, Chang AH, Goh SH, Chow AW: Induction of tumor necrosis factor and interleukin-1 by purified staphylococcal toxic shock syndrome toxin 1 requires the presence of both monocytes and T lymphocytes. Infect Immun 60:2612–2618, 1992
Gjorloff A, Fischer H, Hedlung G, Hansson J, Kenney JS, Allison AC, Sjogren HO, Dohlsten M: Induction of interleukin-1 in human monocytes by the superantigen Staphylococcal enterotoxin A requires the participation of T cells. Cell Immunol 137:61–71, 1991
Parsonnet J, Gillis ZA: Production of TNF by human monocytes in response to toxic-shock-syndrome toxin-1. J Infect Dis 158:1026–1033, 1988
Trede NS, Chantila T, Geha R: AP-1 is stimulated by microbial superantigens in human monocytic cells. Eur J Immunol 23:2129–2135, 1993
Palkama T, Hurme M: Signal transduction mechanisms of HLA-DR-mediated interleukin-1β production in human MØ. Hum Immunol 36:259–267, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szabo, G., Mandrekar, P., Verma, B. et al. Acute ethanol consumption synergizes with trauma to increase monocyte tumor necrosis factor α production late postinjury. J Clin Immunol 14, 340–352 (1994). https://doi.org/10.1007/BF01546318
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01546318