Summary
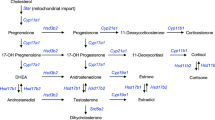
We previously reported that subcutaneous injection of DHEA (5-androsten-3 β-ol-17-one, dehydroepiandrosterone) protected mice from lethal infection. This included both a lethal herpes virus type 2 encephalitis and a lethal systemic coxsackievirus B 4 (CB 4) infection. Androstenediol (5-andros-ten-3 β-17 β-diol, AED), a metabolic product of DHEA is up to 100×more effective in regulating systemic resistance against lethal infection with CB 4 than its precursor DHEA. Compared to DHEA, treatment with AED was markedly superior in protecting mice against virus induced myocardiopathy, pancreopathy, and mortality. In addition to its protective effect, AED but not DHEA, induced a 3–4 fold proliferation of the spleen and thymus in virus infected animals; this effect of AED was only seen above a certain threshold dose. Neither steroid, however, has shown any significant direct antiviral effect in vitro; similarly, virus tissues titers in vivo are not affected by the hormones. Additionally, both DHEA and AED protected against a lethal infection withEnterococcus faecalis. These observations demonstrate that the steroid hormones DHEA and AED provide a novel approach for prevention and protection of the host from a variety of infectious diseases.
Similar content being viewed by others
References
Ben-Nathan D, Feuerstein G (1990) The influence of cold or isolation stress on resistance of mice to West Nile virus encephalitis. Experientia 46: 285–290
Ben-Nathan D, Lachmi B, Lustig S, Feuerstein G (1991) Protection of dehydroepian-drosterone (DHEA) in mice infected with viral encephalitis. Arch Virol 120: 263–271
Berliner DL, Gallegos AJ (1967) Transformation and conjugation of dehydroepian-drosterone by human skin. J Clin Endocrinol 27: 1214–1218
Berliner DL, Pasqualini JR, Gallegos AJ (1968) The formation of water soluble steroids by human skin. J Invest Dermatol 50: 220–224
Daynes RA, Araneo BA (1990) Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol 19: 2319–2325
Daynes RA, Dudley DJ, Araneo BA (1990) Regulation of murine lymphokines production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 3 synthesis by helper T cells. Eur J Immunol 20: 893–802
Faredin I, Toth I (1975) The metabolism of [4-14C] 5 androstene-3 β, 17 β-diol by normal human skin in vitro. Acta Med Acad Sci Hung 32: 139–152
Faredin I, Fazekas A, Toth I, Juslesz M (1969) Transformation in vitro of [4-14C] dehydroepiandrosterone into 7-oxygenated derivatives by the normal human male and female skin tissue. J Invest Dermatol 52: 357–61
Gavan TL (1979) Microbiology: space, equipment, materials, and techniques. In: Henry JB (ed) Clinical diagnosis and management by laboratory methods, vol 2. WB Saunders, Philadelphia, pp 1570–1571
Grossman CJ (1984) Regulation of the immune system by sex steroids. Endocrine Rev 5: 435–455
Grossmann CJ (1985) Interaction between the gonadal steroids and the immune system. Science 227: 257–260
Heffner JE, Milam M (1990) Inhibition of rabbit lung G6PD by DHEA augments oxidant injury. Am J Respir Cell Mol Biol 2: 257–261
Loria RM, Inge TH, Cook S, Szakal A, Regelson W (1988) Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA). J Med Virol 26: 301–14
Loria RM, Regelson W, Padgett DA (1990) Immune response facilitation and resistance to virus and bacterial infections with dehydroepiandrosterone (DHEA). In: Kalimi M, Regelson W (ed) The biologic role of dehydroepiandrosterone (DHEA). W De Gruyter, Berlin, pp 107–130
Loria RM, Padgett DA, Inge TH, Cook SH, Regelson W, Pascua JR, Dalton HP (1989) Dehydroepiandrosterone as an immune up-regulator. Symp Pharm Roussel Uclaf 9: 24
Loria RM, Montgomery LB, Corey LA, Chinchilli V (1984) Influence of diabetes mellitus heredity on susceptibility of coxsackie virus B 4. Arch Virol 81: 251–262
Ott L (1988) An introduction to statistical methods and data analysis, 3rd edn. PWS, Boston
Rager-Zisman B, Allison AC (1973) Effects of immunosuppression on coxsackie B 3 virus infection in mice, and passive protection by circulating antibody. J Gen Virol 19: 339–351
Toth I, Faredin I (1983) Concentrations of androgens and C 19-steroids sulfates in the abdominal skin of healthy women and men. Acta Med Hung 42: 13–20
Woodruff JF, Woodruff JJ (1975) The effects of viral infection on the function of the immune system. In: Notkins AL (ed) Viral immunology and immunopathology. Academic Press, New York, pp 393–418
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loria, R.M., Padgett, D.A. Androstenediol regulates systemic resistance against lethal infections in mice. Archives of Virology 127, 103–115 (1992). https://doi.org/10.1007/BF01309578
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309578