Summary
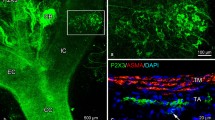
We determined the number, distribution, size, and morphology of paraganglia near the glossopharyngeal, vagus, and sympathetic nerves of rats. The location of paraganglia was revealed by a method that takes advantage of the comparatively high permeability of their blood vessels to Evans blue dye. Rats were fixed by vascular perfusion of glutaraldehyde 2 min after receiving an intravenous injection of Evans blue dye. Paraganglia appeared as circumscribed, intensely blue structures that were readily distinguished from unstained nerves associated with them. Similarly, some groups of small intensely fluorescent (SIF) cells in autonomic and sensory ganglia were surrounded by Evans blue at a time that other portions of the ganglia contained little detectable dye. An average of 92.5 (range 41–134) paraganglia and 41 (range 17–68) blue spots in ganglia were found in the neck, thorax and abdomen of each of 10 rats. Carotid bodies had a mean length of 601 ± 123 μm, width of 275 ± 65 μm, and volume of 25.1 ± 11.2,μm3 × 106. Other paraganglia had an average length of 168 ± 108μm, width of 77 ± 41 μm, and volume of 0.87 ± 1.55 μm3 × 106. The total volume of paraganglion tissue averaged 128 μm3 × 106 (range 62–215 μm3 × 106), 59% of which was due to paraganglia other than the carotid bodies.
By using fluorescence microscopy, we verified that small catecholamine-containing cells, visible because of their yellow-green fluorescence induced by formaldehyde gas, were located in regions along nerves and within ganglia that contained extravascular dye, visible because of its red fluorescence. Electron-microscopic studies confirmed that blue-stained organs (presumptive paraganglia) associated with the superior laryngeal nerve and other branches of the vagus nerve contained cells morphologically similar to glomus cells of the carotid body. Celiac ganglia contained, in addition, some cells similar to chromaffin cells of the adrenal medulla. Paraganglia (but not SIF cells in ganglia) were encapsulated by layers of perineurium, which may constitute a barrier to diffusion. Tortuous thin-walled blood vessels, some with a fenestrated endothelium, were present in all paraganglia examined and were near most groups of SIF cells in ganglia. Neural connections of the small catecholamine-containing cells varied. Most nerve terminals on cells in paraganglia resembled sensory nerve endings on glomus cells of the carotid body, although some were morphologically similar to preganglionic nerves on chromaffin cells of the adrenal medulla.
Similar content being viewed by others
References
Alfes, H., Kindler, J., Knoche, H., Matthiessen, D., Möllmann, H. &Pagnucco, R. (1977) Biogenic amines in the carotid body.Progress in Histochemistry and Cytochemistry 10, 1–69.
Allen, T. H. &Orahovats, P. D. (1950) Combination of toluidine dye isomers with plasma albumin.American Journal of Physiology 161, 473–82.
Anderson, A. O. &Anderson, N. D. (1975) Studies on the structure and permeability of the microvasculature in normal rat lymph nodes.American Journal of Pathology 80, 387–418.
Andrews, W. H. H., Deane, B. M., Howe, A. &Orbach, J. (1971) Abdominal chemoreceptors in the rat.Journal of Physiology 222, 84–5P.
Böck, P. (1970a) Die Feinstruktur der peribronchialen Mikroparaganglien beim Meerschweinchen.Zeitschrift für Zellforschung und mikroskopische Anatomie 110, 243–57.
Böck, P. (1970b) Die Feinstruktur des paraganglionären Gewebes im Plexus suprarenalis des Meerschweinchens.Zeitschrift für Zellforschung und mikroskopische Anatomie 105, 389–404.
Böck, P. (1974) Das System der chromaffinen Paraganglien.Weiner klinische Wochenschrift 86, 95–7.
Böck, P. &Gorgas, K. (1976) Catecholamines and granule content of carotid body type I-cells. InChromaffin, Enterochromaffin and Related Cells (edited byCoupland, R. E. andFujita, T.), pp. 355–74. Amsterdam: Elsevier.
Brightman, M. W. (1967) The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice.Progress in Brain Research 29, 19–37.
Brightman, M. W. &Reese, T. S. (1969) Junctions between intimately apposed cell membranes in the vertebrate brain.Journal of Cell Biology 40, 648–77.
Chen, I. L. &Yates, R. D. (1970) Ultrastructural studies of vagal paraganglia in Syrian hamsters.Zeitschrift für Zellforschung und mikroskopische Anatomie 108, 309–23.
Chiarini, C. N. (1965) Richerche anatomiche sull'innervazione delle zone pressorecettive seno-carotidee, aortiche e polmonari nel ratto.Archivio Italiano di Anatomia e di Embriologia 70, 237–52.
Chiba, T., Black Jr., A. C. &Williams, T. H. (1977) Evidence for dopamine-storing interneurons and paraneurons in rhesus monkey sympathetic ganglia.Journal of Neurocytology 6, 441–53.
Chiba, T. (1978) Monoamine fluorescence and electron microscopic studies on small intensely fluorescent (granule-containing) cells in human sympathetic ganglia.Journal of Comparative Neurology 179, 153–68.
Clasen, R. A., Pandolfi, S. &Hass, G. M. (1970) Vital staining, serum albumin and the blood-brain barrier.Journal of Neuropathology and Experimental Neurology 29, 266–84.
Clementi, F. &Palade, G. E. (1969) Intestinal capillaries I. Permeability to peroxidase and ferritin.Journal of Cell Biology 41, 33–58.
Coleridge, H., Coleridge, J. C. G. &Howe, A. (1967) A search for pulmonary arterial chemoreceptors in the cat, with a comparison of the blood supply of the aortic bodies in the new-born and adult animal.Journal of Physiology 191, 353–74.
Coupland, R. E. (1965a) Electron microscopic observations on the structure of the rat adrenal medulla. I. The ultrastructure and organization of chromaffin cells in the normal adrenal medulla.Journal of Anatomy 99, 231–54.
Coupland, R. E. (1965b)The Natural History of the Chromaffin Cell. London: Longmans Green.
Deane, B. M., Howe, A. &Morgan, M. (1975) Abdominal vagal paraganglia: distribution and comparison with carotid body, in the rat.Acta Anatomica 93, 19–28.
Dretzki, J. (1971) Licht- und elektronenmikroskopische Untersuchungen zum Problem der Blut-Hirn-Schranke circumventricularer Organe der Ratte nach Behandlung mit Myofer.Zeitschrift für Anatomie und Entwicklungsgeschichte 134, 278–97.
Elfvin, L. G. (1968) A new granule-containing nerve cell in the inferior mesenteric ganglion of the rabbit.Journal of Ultrastructure Research 22, 37–44.
Eränkö, O. (1976) (editor)SIF Cells: Structure and Function of the Small, Intensely Fluorescent Sympathetic Cells. Fogarty International Center Proceedings No. 30, Washington, D.C.: U.S. Government Printing Office.
Eränkö, O. (1978) Small intensely fluorescent (SIF) cells and nervous transmission in sympathetic ganglia.Annual Review of Pharmacology and Toxicology 18, 417–30.
Eränkö, O. &Eränkö, L. (1971) Small, intensely fluorescent granule-containing cells in the sympathetic ganglion of the rat.Progress in Brain Research 34, 39–51.
Falck, B. &Owman, C. (1965) A detailed methodological description of the fluorescence method for the cellular demonstration of biogenic monoamines.Acta Universitatis Lundensis 2, 1–23.
Freedman, F. B. &Johnson, J. A. (1969) Equilibrium and kinetic properties of the Evans blue-albumin system.American Journal of Physiology 216, 675–81.
Goormaghtigh, N. (1936) On the existence of abdominal vagal paraganglia in the adult mouse.Journal of Anatomy (London) 71, 77–90.
Grigor'eva, T. A. (1962)The Innervation of Blood Vessels. New York: Pergamon Press.
Grillo, M. A., Jacobs, L. &Comroe J. H., Jr (1974) A combined fluorescence histochemical and electron microscopic method for studying special monoamine-containing cells (SIF cells).Journal of Comparative Neurology 153, 1–14.
Hansen, J. T. &Yates, R. D. (1975) Light, fluorescence and electron microscopic studies of rabbit subclavian glomera.American Journal of Anatomy 144, 477–90.
Hervonen, A., Partanen, S., Vaalasti, A., Partanen, M., Kanerva, L. &Alho, H. (1978) The distribution and endocrine nature of the abdominal paraganglia of adult man.American Journal of Anatomy 153, 563–72.
Hill, C. E., Watanabe, H. &Burnstock, G. (1975) Distribution and morphology of amphibian extra-adrenal chromaffin tissue.Cell and Tissue Research 160, 371–87.
Hollinshead, W. H. (1940) Chromaffin tissue and paraganglia.Quarterly Review of Biology 15, 156–71.
Hollinshead, W. H. (1941) Chemoreceptors in the abdomen.Journal of Comparative Neurology 74, 269–85.
Hollinshead, W. H. (1946) The function of the abdominal chemoreceptors of the rat and mouse.American Journal of Physiology 147, 654–60.
Howe, A. (1956) The vasculature of the aortic bodies in the cat.Journal of Physiology 134, 311–18.
Howe, A. &Pack, R. J. (1977) The response of abdominal vagal fibres in the rat to changes in inspired oxygen concentration.Journal of Physiology 270, 37–38P.
Hueper, W. C. &Ichniowski, C. T. (1944) Toxicopathologic studies on the dye T-1824.Archives of Surgery 48, 17–26.
Hughes, T. (1965) Portal blood supply to glomus tissue and its significance.Nature 205, 149–51.
Ivanov, D. P. (1974) Recherches ultrastructurales sur les cellules paraganglionnaires du ganglion coeliaque du rat et leurs connexions avec les neurones.Acta Anatomica 89, 266–86.
Jacobowitz, D. (1970) Catecholamine fluorescence studies of adrenergic neurons and chromaffin cells in sympathetic ganglia.Federation Proceedings 29, 1929–44.
Kjaergaard, J. (1973)Anatomy of the Carotid Glomus and Carotid Glomus-like Bodies (Non-chromaffin Paraganglia). Copenhagen: F.A.D.L.'s Forlag.
Kleinsasser, O. (1964) Das Glomus laryngicum inferior.Archiv Ohren-, Nasen- und Kehlkopfheilk 184, 214–24.
Kobayashi, S. (1971) Comparative cytological studies of the carotid body, 1. Demonstration of monoamine-storing cells by correlated chromaffin reaction and fluorescence histochemistry.Archivum histologicum japonicum 33, 319–39.
Kohn, A. (1903) Die Paraganglien.Archiv für mikroskopische Anatomie 62, 263–365.
Laidler, P. &Kay, J. M. (1975) A quantitative morphological study of the carotid bodies of rats living at a simulated altitude of 4300 metres.Journal of Pathology 117, 183–91.
Landis, E. M. (1964) Heteroporosity of the capillary wall as indicated by cinematographic analysis of the passage of dyes.Annals of the New York Academy of Sciences 116, 765–73.
Lempinen, M. (1964) Extra-adrenal chromaffin tissue of the rat and the effect of cortical hormones on it.Acta Physiologica Scandinavica 62, Suppl. 231, 1–91.
LeVeen, H. H. &Fishman, W. H. (1947) Combination of Evans blue with plasma protein: its significance in capillary permeability studies, blood dye disappearance curves, and its use as a protein tag.American Journal of Physiology 151, 26–33.
Levick, J. R. &Michel, C. C. (1973) The effect of bovine albumin on the permeability of frog mesenteric capillaries.Quarterly Journal of Experimental Physiology 58, 87–97.
Majno, G. &Palade, G. E. (1961) Studies on inflammation I. The effect of histamine and serotonin on vascular permeability: an electron microscopic study.Journal of Biophysical and Biochemical Cytology 11, 571–605.
Malmgren, L. T. &Olsson, Y. (1980) Differences between the peripheral and the central nervous system in permeability to sodium fluorescein.Journal of Comparative Neurology 191, 103–17.
Mascorro, J. A. &Yates, R. D. (1973) Fine structural comparisons between paraganglion and adrenal medullary cells in the Syrian hamster.Texas Reports on Biology and Medicine 31, 519–35.
Mascorro, J. A. &Yates, R. D. (1974) Innervation of abdominal paraganglia: an ultrastructural study.Journal of Morphology 142, 153–63.
Mascorro, J. A. &Yates, R. D. (1975) A review of abdominal paraganglia: ultrastructure, mitotic cells, catecholamine release, innervation, light and dark cells, vascularity. InElectron Microscopic Concepts of Secretion: Ultrastructure of Endocrinal and Reproductive Organs (edited byHess, M.), pp. 435–52. New York: Wiley.
Mascorro, J. A. &Yates, R. D. (1977) The anatomical distribution and morphology of extra-adrenal chromaffin tissue (abdominal paraganglia) in the dog.Tissue and Cell 9, 447–60.
Mascorro, J. A., Yates, R. D. &Chen, I. -L. (1976) A glutaraldehyde/potassium dichromate tracing method for the localization and preservation of abdominal extra-adrenal chromaffin tissues.Stain Technology 50, 391–6.
Matthews, M. R. &Raisman, G. (1969) The ultrastructure and somatic efferent synapses of small granule-containing cells in the superior cervical ganglion.Journal of Anatomy 105, 255–82.
McDonald, D. M. (1981) Peripheral chemoreceptors: structure-function relationships of the carotid body. InRespiratory Control and Clinical Applications, Volume 17 (Part I), of:Lung Biology in Health and Disease (edited byHornbein, T. F.), pp. 105–319. New York: Marcel Dekker. Inc.
McDonald, D. M. &Mitchell, R. A. (1975) The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: a quantitative ultrastructural analysis.Journal of Neurocytology 4, 177–230.
McEwen, L. M. (1956) The effect on the isolated rabbit heart of vagal stimulation and its modification by cocaine, hexamethonium and ouabain.Journal of Physiology 131, 678–89.
Morgan, M., Pack, R. J. &Howe, A. (1976) Structure of cells and nerve endings in abdominal vagal paraganglia of the rat.Cell and Tissue Research 169, 467–84.
Nonidez, J. F. (1935) The aortic (depressor) nerve and its associated epithelioid body, the glomus aorticum.American Journal of Anatomy 57, 259–301.
Olsson, Y. (1968) Topographical differences in the vascular permeability of the peripheral nervous system.Acta Neuropathologica 10, 26–33.
Olsson, Y. &Hossmann, K. -A. (1970) Fine structural localization of exudated protein tracers in the brain.Acta Neuropathologica 16, 103–16.
Olsson, Y. &Reese, T. S. (1971) Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy.Journal of Neuropathology and Experimental Neurology 30, 105–19.
Pearse, A. G. E. (1968)Histochemistry Theoretical and Applied, Volume I, Baltimore: Williams and Wilkins.
Rawson, R. A. (1942–43) The binding of T-1824 and structurally related diazo dyes by the plasma proteins.American Journal of Physiology 138, 708–17.
Reese, T. A. &Brightman, M. W. (1968) Similarity in structure and permeability to peroxidase of epithelia overlying fenestrated cerebral capillaries.Anatomical Record 160, 414 (abstract).
Richardson, K. C. (1969) The fine structure of autonomie nerves after vital staining with methylene blue.Anatomical Record 164, 359–78.
Rous, P., Gilding, H. P. &Smith, F. (1930) The gradient of vascular permeability.Journal of Experimental Medicine 51, 807–30.
Santer, R. M., Lu, K. -S., Lever, J. D. &Presley, R. (1975) A study of the distribution of chromaffin-positive (CH+) and small intensely fluorescent (SIF) cells in sympathetic ganglia of the rat at various ages.Journal of Anatomy 119, 589–99.
Schen, R. J., Rabinovitz, M., Goldschmid, A. &Tenne, M. (1967) The affinity of Evans blue for the α1-lipoprotein of human serum.Clinica Chimica Acta 16, 445–8.
Sear, H., Allen, T. H. &Gregersen, M. I. (1953) Simultaneous measurement,in dogs of plasma volume with I131 human albumin and T-1824 with comparisons of their long-term disappearance from the plasma.American Journal of Physiology 175, 240–2.
Siegel, S. (1956)Nonparametric Statistics for the Behavioral Sciences, pp. 127–31. New York: McGraw-Hill.
Steinwall, O. &Klatzo, I. (1966) Selective vulnerability of the blood-brain barrier in chemically induced lesions.Journal of Neuropathology and Experimental Neurology 25, 542–59.
Taxi, J. (1979) The chromaffin and chromaffin-like cells in the autonomie nervous system.International Review of Cytology 57, 283–343.
Watzka, M. (1966) Über die Paraganglien in der Plica ventricularis des menschlichen Kehlkopfes.Acta Anatomica 63, 300–8.
Wilde, W. S., Hill, J. H., Wilson E. &Schielke, G. P. (1971) Exchange of free and albumin-bound Evans blue in interstitium of hamster kidney.American Journal of Physiology 220, 1991–9.
Yates, R. D., Mascorro, J. A., Hansen, J. T., &Chen, I. -L. (1976) Comparison of the structure of carotid and subclavian bodies and abdominal paraganglia. InSIF Cells: Structure and function of the Small, Intensely fluorescent Sympathetic Cells, Fogarty International Center Proceedings No. 30 (edited byEränkö, O.), pp. 54–65. Washington D.C.: U.S Government Printing Office.
Zar, J. H. (1974)Biostatistical Analysis. New Jersey: Prentice-Hall.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McDonald, D.M., Blewett, R.W. Location and size of carotid body-like organs (paraganglia) revealed in rats by the permeability of blood vessels to Evans blue dye. J Neurocytol 10, 607–643 (1981). https://doi.org/10.1007/BF01262593
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01262593