Abstract
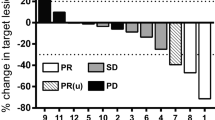
Nineteen patients with recurrent high grade gliomas were treated in a phase I/II trial with aggressive debulking of the tumor, mitogen activated IL-2 stimulated peripheral blood lymphocytes (MAK cells), and rIL-2. Phytohemagglutin (PHA) was introduced into the tumor site in 16 patients prior to implanting MAK cells and IL-2 in an attempt to trigger more effective lysis of the tumorin vivo. In vitro both TNF bioactivity and cytolytic activity of long term cultured MAK (LMAK) cells were dramatically enhanced by adding PHA to the cultures of these activated PBL. Three of eleven patients (27%) had a decrease in size of the enhancing lesion on CT and/or MRI. Seven (37%) patients clinically improved. Median survival after therapy was 30 weeks. PHA was shown to be safein vivo and more effective than IL-2 triggering enhanced effector functionin vitro.
Similar content being viewed by others
References
Chong ASF, Aleksijevic A, Scuderi P, Hersh EM, Grimes WJ: Phenotypic and functional analysis lymphokine-activated killer (LAK) cell clones. Cancer Immunol Immunother 29: 270–278, 1989
Grimm EA, Rosenberg SA: The human lymphokine-activated killer cell phenomenon. Lymphokines 9: 279–311, 1984
Kalland T, Belfrage H, Bhiladvala P, Hedlund G: Analysis of the murine lymphokine-activated killer (LAK) cell phenomenon: dissection of effectors and progenitors into NK- and T-like cells. J Immunol 138: 3640–3645, 1987
Yamamoto R, Coss J, Vayuvegula B, Gupta S, Beamer Y, Jacques S, Jeffes E, Carson W, Jakowatz J, Granger G: Generation of stimulated, lymphokine activated T killer (T-LAK) cells from the peripheral blood of normal donors and adult patients with recurrent glioblastoma. J Immunological Methods 137: 225–235, 1991
George RE, Loudon WG, Moser RP, Bruner JM, Steck PA, Grimm EA:In vitro cytolysis of primitive neuroectodermal tumors of the posterior fossa (medulloblastoma) by lymphokine-activated killer cells. J Neurosurg 69:403–409, 1988
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze ST, Yany JC, Seipp C, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE: Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. New Eng J Med 319: 1676–1680, 1988
Rosenberg SA, Spiess P, Lafreniere R: A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233: 1318–1321, 1986
Lafreniere R, Rosenberg SA: Adoptive immunotherapy of murine hepatic metastases with lymphokine activated killer cells and recombinant interleukin 2 can mediate the regression of both immunogeneic and nonimmunogenic sarcomas and an adenocarcinoma. J Immunol 135: 4273–4280, 1985
Mazumder A, Rosenberg SA: Successful immunotherapy of natural killer-resistant established pulomnary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activatedin vitro by interleukin 2. J Exp Med 159: 495–507, 1984
Mule JJ, Shu S, Schwartz SL, Rosenberg SA: Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science 225: 1487–1489, 1984
Mule JJ, Shu S, Rosenberg SA: The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin-2in vivo. J Immunol 135: 646–652, 1985
Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, Seipp CA, Simpson CG, White DE: A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 316: 889–897, 1987
Takai N, Tanaka R, Yoshida S, Hara N, Takafumi S:In vivo andin vitro effect of adoptive immunotherapy of experimental murine brain tumors using lymphokine-activated killer cells. Cancer Res 48: 2047–2052, 1988
VanHaelst-Pisani CM, Pisani RJ, Kovach JS: Cancer immunotherapy: current status of treatment with interleukin 2 and lymphokine-activated killer cells. Mayo Clin Proc 64: 451–465, 1989
Kornblith PL, Walker MD, Cassady JR (eds): General principles of treatment, in Neurologic Oncology, Philadelphia, J.B. Lippincott Company, 1987, pp 93–113
Saloman M: Malignant glioma management. Neurosurg Clin N A 1: 49–63, 1990
Saloman M: Survival in Glioblastoma: Historical Perspective. Neurosurgery 7: 435–439, 1980
Carson W, Jakowatz J, Yamamoto R, Fitzgerald T, Gupta S, Vayuvegula B, Lucci J, Beckman M, Dulkanchainun S, Granger G, Jeffes E: Rat mitogen-stimulated lymphokine-activated (MAK) T killer cells: Production and effects on C6 Glioma Cellin vitro andin vivo in the brain of Wistar Rats J. of Immunotherapy 10: 131–140, 1991
Ingram M, Jacques S, Freshwater DB, Techy GB, Shelden CH, Helsper JT: Salvage immunotherapy of malignant glioma. Arch Surg 122: 1483–1486, 1987
Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA: Interleukin-2 and autologous lymphokine-activated killer cells in the treatment of malignant glioma. J Neurosurg 64: 743–749, 1986
Merchant RE, Merchant LH, Cook SHS, McVical DW, Young HF: Intralesional infusion of lymphokine-activated killer (LAK) cells and recombinant interleukin-2(rIL-2) for the treatment of patients with malignant brain tumor. Neurosurgery 23: 725–732, 1988
Yoshida S, Tanaka R, Takai N, Ono K: Local administration of autologous lymphokine-activated killer cells and recombinant interleukin 2 to patients with malignant brain tumors. Cancer Res 48: 5011–5016, 1988
Dumas-Duport C, Scheithauer B, O'Fallon J, Kelley P: Grading of Astrocytomas: A simple and reproducible method. Cancer 62: 2152–2165, 1988
Burger P, Vogel F, Green S, Strike T: Glioblastoma multiforme and anaplastic astrocytoma. Cancer 56: 1106–1111, 1985
Jeffes E, Schmitz K, Yamamoto R, Tomich J, Beckman M, Nep R, Knauer M: A simple nonisotopicin vitro bioassay for LT and TNF employing sodium fluoride treated L-929 target cells which detects picogram quantities of LT and TNF and is as sensitive as TNF assays done with ELISA methodology, Lymphokine Research 10: 147–151, 1991
Hiserodt JC, Tiangco GJ, Granger GA: The LT system in experimental animals: I. Rapid release of high levels of LT activity from murine lymphocytes during interaction with lectin-treated allogeneic or xenogeneic target cellsin vitro. J Immunol 123: 311–316, 1979
Granger GA, Daynes RA, Runge PE, Prieur AM, Jeffes EWB: Lymphocyte effector molecules and cell-mediated immune reactions. Contemporary Topics in Molecular Immunology 4: 205–241, 1975
Young B, Oldfield E, Markesbery W, Haack D, Tibbs P, McCombs P, Chin H, Maruyama Y, Meacham W: Reoperation for glioblastoma. J Neurosurg 55: 917–921, 1981
Wetzel N, Anderson M, Sheilds T: Pulmonary embolism as a cause of death in the neurosurgical patient. J Neurosurg 17: 664–668, 1960
Kayser-Gatchalian MC, Kayser K: Thrombosis and intracranial tumors. J Neurol 209: 217–224, 1975
Sawaya R, Decourteen-Meyers G, Copeland B: Massive preoperative pulmonary embolism and suprasellar brain tumor: case report and review of the literature. Neurosurgery 15: 566–571, 1984
Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA: Lymphokine-activated killer cell phenomenon: lysis of natural killer-resistant fresh solid tumor cells by interleukin-2 activated autologous human peripheral blood lymphocytes. J Exp Med 155: 1823–1841, 1982
Kruse CA, Mitchell DH, Lillehei KO, Johnson SD, McCleary EL, Moore GE, Waldrop S, Mierau W: Interleukin-2 activated lymphocytes from brain tumor patients. Cancer 64: 61–69, 1989
Hiserodt JC, Granger GA: Inhibition of human lymphocyte-mediated mitogen-induced cytotoxicity of murine L-929 cells by heterologous anti-human lymphotoxin anitserain vitro. J Immunol 119: 374–380, 1977
Mazumder A, Grimm EA, Rosenberg SA: Characterization of the lysis of fresh human solid tumors by autologous lymphocytes activatedin vitro with phytohemagglutinin. J Immunol 130: 958–964, 1983
Barba D, Saris SC, Holder C. Rosenberg SA, Oldfield EH: Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg 70: 175–182, 1989
Ingram M, Buckwalter JG, Jacques DB, Freshwater DB, Abts RM, Techy GB, Miyagi K, Shelden CH, Rand RW, English LW: Immunotherapy for recurrent malignant glioma: an interim report on survival. Neurological Res 12: 265–273, 1990
Harris PC, Yamamoto RS, Crane J, Granger GA: The human LT system. X The initial form released by T-enriched lymphocytes is 1500 m.w., associated with small nonlytic components, and can dissociate into alpha, beta, and gamma m.w. classes. J Immunology 126: 2165–2170, 1981
Hiserodt JC, Tiangco GJ, Granger GA: The LT system in experimental animals: II. Physical and immunologic characteristics of molecules with LT activity rapidly release by murine lymphoid cells activated on lectin-coated allogeneic monolayersin vitro. J Immunol 123: 317–324, 1979
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jeffes, E.W.B., Beamer, Y.B., Jacques, S. et al. Therapy of recurrent high grade gliomas with surgery, and autologous mitogen activated IL-2 stimulated killer (MAK) Lymphocytes: I. Enhancement of MAK lytic activity and cytokine production by PHA and clinical use of PHA. J Neuro-Oncol 15, 141–155 (1993). https://doi.org/10.1007/BF01053935
Issue Date:
DOI: https://doi.org/10.1007/BF01053935