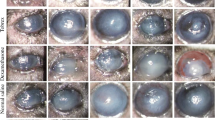
Abstract
In order to identify the causative factors of ring abscess, which is the characteristic feature of pseudomonal keratitis, pseudomonal endotoxin, exotoxin A, and elastase were each separately injected into guinea pig cornea. There was no formation of ring abscess. Injection of livingPseudomonas aeruginosa strains IFO3455 and Takamatsu which produce all three molecules, clearly induced ring abscess. In contrast, when heat-killed bacteria strain IFO3455 or living bacteria of the non-elastase-producing strain PA103 were injected, ring abscess was not induced. Furthermore, when living bacteria strain IFO3455 were injected with anti-elastase antibody or a protease inhibitor, ovomacroglobulin, ring abscess formation was significantly inhibited. Histological examination demonstrated that the ring abscess was a dense accumulation and aggregation of polymorphonuclear leukocytes (PMN) with debris of cells and lamellae in the deep stroma at the corneal margins, suggesting prevention of PMN migration to the central lesion. The presence of anti-elastase antibody or a specific elastase inhibitor facilitated PMN migration towards living bacteria strain IFO3455 in an in vitro model. These results indicate that pseudomonal elastase is a necessary but not sufficient factor in the formation of ring abscess in pseudomonal keratitis.
Similar content being viewed by others
References
Ando E, Ando Y, Inoue M, Morino Y, Kamata R, Okamura R (1990) Inhibition of corneal inflammation by an acylated superoxide dismutase derivative. Invest Ophthalmol Vis Sci 31:1963–1967
Böyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Scand J Lab Invest 21:77–89
Butrus SI, Klotz SA (1990) Contact lens surface deposits increase the adhesion ofPseudomonas aeruginosa. Curr Eye Res 9:717–725
Butrus SI, Klotz SA, Misra RP (1987) The adherence ofPseudomonas aeruginosa to soft contact lenses. Ophthalmology 94:1310–1314
Chenoweth DE, Rowe JG, Hughli TE (1979) A modified method for chemotaxis under agarose. J Immunol Methods 25:337–353
Chusid MJ, Davis SD (1985) Polymorphonuclear leukocyte kinetics in experimentally induced keratitis. Arch Ophthalmol 103:270–275
Cryz SJ, Pitt TL, Furer E, Germanier R (1984) Role of lipopolysaccharide in virulence ofPseudomonas aeruginosa. Infect Immun 44:508–513
Donnenfield ED, Cohen EJ, Arentsen JJ, Genvert GI, Laibson PR (1986) Changing trends in contact lens associated corneal ulcers: an overview of 116 cases. CLAD J 12:145–149
Döring G, Goldstein W, Röll A, Schiotz PO, Høiby N, Botzenhart K (1985) Role ofPseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun 49:557–562
Hedberg M, Miller JK, Tomkins VN (1969) Elastase activity ofPseudomonas aeruginosa isolates from hospital patients. Am J Clin Pathol 52:631–633
Hogan MJ, Zimmerman LE (1968) Diffuse ocular disease and its sequelae. In: Hogan MJ, Zimmerman LE (eds) Ophthalmic pathology: an atlas and textbook. Saunders, Philadelphia, pp 96–136
Howe TR, Iglewski BH (1984) Isolation and characterization of alkaline protease-deficient mutants ofPseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun 43:1058–1063
Iglewski BH, Burns RP, Gipson IK (1977) Pathogenesis of corneal damage fromPseudomonas exotoxin A. Invest Ophthalmol Vis Sci 16:73–76
Kawaharajo K, Abe C, Homma JY, Kawano M, Gotoh E, Tanaka N, Morihara K (1974) Corneal ulcers caused by protease and elastase fromPseudomonas aeruginosa. Jpn J Exp Med 44:435–442
Kawaharajo K, Homma JY (1975) Pathogenesis of the mouse keratitis produced withPseudomonas aeruginosa. Jpn J Exp Med 45:515–524
Kessler E, Kennah HE, Brown SJ (1977)Pseudomonas protease: Purification, partial characterization and its effect on collagen, proteoglycan and rabbit corneas. Invest Ophthalmol Vis Sci 16:488–497
Kharazmi A (1989) Interactions ofPseudomonas aeruginosa protease with the cells of the immune system. Antibiot Chemother 42:169–176
Kharazmi A, Döring G, Høiby N, Valerius NH (1984) Interaction ofPseudomonas aeruginosa alkaline protease and elastase with human polymorphonuclear leukocytes in vitro. Infect Immun 43:161–165
Kreger AS, Gray LD (1978) Purification ofPseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun 19:630–648
Kreger AS, Lyerly DM, Hazlett LD, Berk RS (1986) Immunization against experimentalPseudomonas aeruginosa andSerratia marcescens keratitis. Invest Ophthalmol Vis Sci 27:932–939
Laibson PR (1972) Annual review. Cornea and sclera. Arch Ophthalmol 88:553–574
Miura F (1991) The mechanism of corneal ring formation caused by bacterial endotoxin. Acta Soc Ophthalmol Jpn 95:625–634
Miyagawa S, Kamata R, Matsumoto K, Okamura R, Maeda H (1991) Inhibitory effects of ovomacroglobulin on bacterial keratitis in rabbits. Graefe's Arch Clin Exp Ophthalmol 229:281–286
Molla A, Matsumura Y, Yamamoto T, Okamura R, Maeda H (1987) Pathogenic capacity of proteases fromSerratia marcescens andPseudomonas aeruginosa and their suppression by chicken egg white ovomacroglobulin. Infect Immun 55:2509–2517
Mondino BJ, Rabin BS, Kessler E, Gallo J, Brown SI (1977) Corneal rings with gram-negative bacteria. Arch Ophthalmol 95:2222–2225
Morihara K, Homma JY (1985)Pseudomonas proteases. In: Holder IA (ed) Bacterial enzymes and virulence. CRC Press, Boca Raton, Fla, pp 41–79
Mull JD, Callahan WS (1965) The role of the elastase ofPseudomonas aeruginosa in experimental infection. Exp Mol Pathol 4:567–575
Nelson RD, Quie PG, Simmons RL (1975) Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocyte. J Immunol 115:1650–1656
Nishino N, Powers JC (1980)Pseudomonas aeruginosa elastase: development of a new substrate, inhibitors, and affinity ligands. J Biol Chem 255:3482–3486
Ohman DE, Burns RP, Iglewski BH (1980) Corneal infections in mice with toxin A and elastase mutants ofPseudomonas aeruginosa. J Infect Dis 142:547–555
Okamoto T, Ueda K, Kambara T, Kutsuna T (1986) Chemotactic activity for polymorphonuclear and mononuclear leukocytes in rheumatoid synovial fluids. Acta Pathol Jpn 36:1109–1122
Onodera T, Kumagai S, Watanabe T, Tazawa Y, Hirata M, Yoshida M (1985) Experimental rabbit corneal ring formation caused by bacterial endotoxin. Acta Soc Ophthalmol Jpn 91:465–472
Onodera T (1987) The mechanism of corneal ring formation caused by endotoxin. Acta Soc Ophthalmol Jpn 91:465–472
Ormerod LD, Hertzmark E, Gomez DS, Stabiner RG, Schanzlin DJ, Smith RE (1987) Epidemiology of microbial keratitis in southern California. Ophthalmology 94:1322–1333
Ouchterlony O (1953) Diffusion in gel methods for immunological analysis. Prog Allergy 5:1–78
Siefferman CM, Regelmann WE, Gray BH (1991)Pseudomonas aeruginosa variants isolated from patients with cystic fibrosis are killed by a bactericidal protein from human polymorphonuclear leukocytes. Infect Immun 59:2152–2157
Steuhl KP, Döring G, Henni A, Thiel HJ, Botzenhart K (1987) Relevance of host-derived and bacterial factors inPseudomonas aeruginosa corneal infections. Invest Ophthalmol Vis Sci 28:1559–1568
Sugihara S, Yamamoto T, Tsuruta J, Tanaka J, Hiraoka T, Tashiro S, Miyauchi Y, Kambara T (1991) Enzyme-induced aggregation and disaggregation of tumor cells via the cell surface glycocalyxin association with deoxyribonucleic acid. Acta Pathol Jpn 41:327–335
Twining SS, Davis SD, Hyndiuk RA (1986) Relationship between proteases and descemetocele formation in experimentalPseudomonas keratitis. Cur Eye Res 5:503–510
Twining SS, Lohr KM, Moulder JE (1986) The immun system in experimentalPseudomonas keratitis. Invest Ophthalmol Vis Sci 27:507–515
Van Horn DL, Davis SD, Hyndiuk RA, Alpren TVP (1978) Pathogenesis of experimentalPseudomonas keratitis in the guinea pig: bacteriologic, clinical, and microscopic observations. Invest Ophthalmol Vis Sci 17:1076–1086
Wilson LA (1983) Bacterial corneal ulcers. In: Duane TD (eds) Clinical ophthalmology, vol 4. Harper & Row, New York, pp 1–16
Wretlind B, Pavlovskis OR (1981) The role of proteases and exotoxin A in the pathogenicity ofPseudomonas aeruginosa infections. J Infect Dis Suppl 29:13–19
Wretlind B, Pavlovskis OR (1983)Pseudomonas aeruginosa elastase and its role inPseudomonas infections. Rev Infect Dis 5:998–1004
Woods DE, Sokol PA, Bryan LE, Storey DG, Mattingly SJ, Vogel HJ, Ceri H (1991) In vivo regulation of virulence inPseudomonas aeruginosa associated with genetic rearrangement. J Inf Dis 163:143–149
Yamamoto T, Cochrane CG (1982) A protease-like permeability factor in guinea pig skin. Am J Pathol 107:127–134
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ijiri, Y., Yamamoto, T., Kamata, R. et al. The role ofPseudomonas aeruginosa elastase in corneal ring abscess formation in pseudomonal keratitis. Graefe's Arch Clin Exp Ophthalmol 231, 521–528 (1993). https://doi.org/10.1007/BF00921117
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00921117