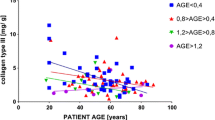
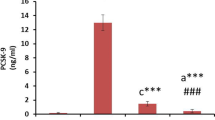
Abstract
Glycosaminoglycans are regular constituents of the arterial wall and essential for its structure and function. The arteriosclerosis-dependent changes of glycosaminoglycans were investigated, the degree of arteriosclerosis was monitored by the cholesterol content of the tissue. Histological characterization was achieved by electron microscopy. Total glycosaminoglycans were isolated from 33 delipidated segments of human aorta thoracica after exhaustive proteolytic digestion, and fractionated into the individual glycosaminoglycans by a multistep purification procedure. Chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), and hyaluronate (HA) were identified and quantified by chemical and enzymatic analysis. The concentration of total and individual glycosaminoglycans, expressed as mg/g delipidated dry weight of tissue, decreased significantly with increasing cholesterol content of tissue (p=0.0005–0.005). The extent of decrease differed between the individual glycosaminoglycans as indicated by a shift in the CS/DS:HA:HS ratio from 47:32:21 in low cholesterol aortic segments to 59:29:12 in cholesterol-rich specimens. Determination of the relative molecular masses (Mr) revealed 58 kDa for CS/DS and 92 kDa for HS with a (statistically not significant) increase of the molecular mass of CS/DS and a decrease of HS with increasing cholesterol content. The copolymeric CS/DS glycosaminoglycans were disintegrated enzymatically into CS and DS containing fragments. A significantly higher relative DS content (p=0.01) was found in cholesterol-rich arterial tissue (32.5%) as compared with low cholesterol tissue samples (28.8%). Cell culture experiments revealed that human arterial HS is able to inhibit the proliferation of cultured human arterial smooth muscle cells. The HS concentration required for a 30% inhibition of smooth muscle cell proliferation was in the same order as the tissue concentration of HS. This confirms the function of HS as an endogenous inhibitor of cell division and its impact for the development of atherosclerosis.
Similar content being viewed by others
References
Bendayan P, Boccalon H, Dupouy D, Boneu B (1994) Dermatan sulfate is a more potent inhibitor of clot bound thrombin than unfractionated and low molecular weight heparins. Thromb Haemost 71: 576–580
Berenson GS, Radhakrishnamurthy B, Srinivasan SR, Vijayagopal P, Dalferes ER (1985) Proteoglycans and potential mechanisms related to atherosclerosis. Ann NY Acad Sci 454: 69–78
Berenson GS, Radhakrishnamurthy B, Srinivasan SR, Vijayagopal P, Dalferes ER (1988) Arterial wall injury and proteoglycan changes in atherosclerosis. Arch Pathol Lab Med 112: 1002–1010
Bittner T, Muir H (1962) A modified uronic acid carbazole reaction. Anal Biochem 4: 330–334
Bocan TMA, Schifani TA, Guyton JR (1986) Ultrastructure of the human aortic fibrolipid lesion. J Pathol 123: 413–424
Bourin MC, Lindahl U (1993) Glycosaminoglycans and the regulation of blood coagulation. Biochem J 289: 313–330
Cardoso LEM, Mourào PAS (1994) Glycosaminoglycan fractions from human arteries presenting diverse susceptibilities to atherosclerosis have different binding affinities to plasma LDL. Arterioscler Thromb 14: 115–124
Cherchi GM, Coinu R, Demuro P, Formato M, Sanna G, Tidore M, Tira ME, De Luca G (1990) Structural and functional modifications of human aorta proteoglycans in atherosclerosis. Matrix 10: 362–372
Dalferes ER, Radhakrishnamurthy B, Ruiz HA, Berenson GS (1987) Compositions of proteoglycans from human atherosclerotic lesions. Exp Mol Pathol 47: 363–376
Davies MJ, Woolf N, Rowles P, Richardson PD (1994) Lipid and cellular constituents of unstable human aortic plaques. Basic Res Cardiol 89 Suppl 1: 33–39
Hollmann J, Schmidt A, von Basewitz DB, Buddecke E (1989) Relationship of sulfated glycosaminoglycans and cholesterol content in normal and arteriosclerotic human aorta. Arteriosclerosis 9: 154–158
Ismail NAE, Alavi MZ, Moore S (1994) Lipoprotein-proteoglycan complexes from injured rabbit aortas accelerate lipoprotein uptake by arterial smooth muscle cells. Atherosclerosis 105: 79–87
Jimi S, Sakata N, Matunaga A, Takebayashi S (1994) Low density lipoproteins bind more to type I and III collagens by negative charge mechanisms than to type IV and V collagen. Atherosclerosis 107: 109–116
Kjellén L, Lindahl U (1991) Proteoglycans: Structure and interactions. Annu Rev Biochem 60: 443–475
Lévesque H, Girard N, Maingonnat C, Delpech A, Chauzy C, Tayot J, Courtois H, Delpech B (1994) Localization and solubilization of hyaluronan and of the hyaluronan binding protein hyaluronectin in human normal and arteriosclerotic arterial walls. Atherosclerosis 105: 51–62
Marcum JA, Atha DH, Fritze LMS, Nawroth P, Stern D, Rosenberg RD (1986) Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem 261: 7507–7517
Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G (1992) Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. J Biol Chem 267: 20435–20443
Murata K, Yokoyama Y (1989) Acidic glycosaminoglycans in human atherosclerotic cerebral arterial tissues. Atherosclerosis 78: 69–79
Nakamura M, Torii S, Yatsuki K, Kikuchi Y, Yamamoto H (1971) Cerebral atherosclerosis in Japanese. — 1. Lipids and glycosaminoglycans in cerebral arteries. Atherosclerosis 13: 185–197
Nakashima Y, Kuroiwa A, Nakamura M (1985) Silicon contents in normal, latty streaks and atheroma of human aortic intima: its relationship with glycosaminoglycans. Br J Exp Pathol 66: 123–127
Riessen R, Isner JM, Blessing E, Loushin C, Nikol S, Wight TN (1994) Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol 144: 962–974
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809
Sambandam T, Baker JR, Christner JE, Ekborg SL (1991) Specifity of the low density lipoprotein-glycosaminoglycan interaction. Arterioscler Thromb 11 (3): 561–568
Schmidt A, Buddecke E (1988) Cell-associated proteoheparan sulfate from bovine arterial smooth muscle cells. Exp Cell Res 178: 242–253
Schmidt A, Lemming G, Yoshida K, Buddecke E (1992) Molecular organization and antiproliferative domains of arterial tissue heparan sulfate. Eur J Cell Biol 59: 322–328
Schmidt A, Skaletz-Rorowski A, Breithardt G, Buddecke E (1995) Growth status-dependent changes of bFGF compartmentalization and heparan sulfate structure in arterial smooth muscle cells. Eur J Cell Biol 67: 130–135
Schmidt A, Yoshida K, Buddecke E (1992) The antiproliferative activity of arterial heparan sulfate resides in domains enriched with 2-O-sulfated uronic acid residues. J Biol Chem 267: 19242–19247
Shworak NW, Shirakawa M, Colliec-Jouault S, Liu J, Mulligan RC, Birinyi LK, Rosenberg RD (1994) Pathway-specific regulation of the synthesis of anticoagulantly active heparan sulfate. J Biol Chem 269 (40): 24941–24952
Stary CH, Chandler AB, Dinsmore RE, Fuster V, Glagow S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the committee on vascular lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92: 1355–1374
Stary HC, Chandler AB, Glagow S, Guyton JR, Insull W Jr, Rosenfeld ME, Schaffer A, Schwartz CJ, Wagner WD, Wissler RW (1994) A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: a report from the committee on vascular lesions of the Council on Arteriosclerosis, American Heart Association. Special report. Arterioscler Thromb 14: 840–856
Stevens RL, Colombo M, Gonzales JJ, Hollander W, Schmid K (1976) The glycosaminoglycans of the human artery and their changes in atherosclerosis. J Clin Invest 58: 470–481
Vijayagopal P (1994) Regulation of the metabolism of lipoprotein-proteoglycan complexes in human monocyte-derived macrophages. Biochem J 301: 675–681
Vijayagopal P, Srinivasan SR, Radhakrishnamurthy B, Berenson GS (1993) Human monocyte-derived macrophages bind low-density-lipoprotein-proteoglycan complexes by a receptor different from the low-density-lipoprotein receptor. Biochem J 289 (Pt3): 837–844
Völker W, Schmidt A, Buddecke E (1987) Mapping of proteoglycans in human arterial tissue. Eur J Cell Biol 45: 72–79
Völker W, Schmidt A, Buddecke E (1989) Cytochemical changes in a human arterial proteoglycan related to atherosclerosis. Atherosclerosis 77: 117–130
Völker W, Schmidt A, Oortmann W, Broszey T, Faber V, Buddecke E (1990) Mapping of proteoglycans in atherosclerotic lesions. Eur Heart J 11: 29–40
Wagner WD (1985) Proteoglycan structure and function as related to atherosclerosis. Ann NY Acad Sci 454: 52–68
Wagner WD, Salisbury BGJ, Rowe HA (1986) A proposed structure of chondroitin 6-sulfate proteoglycan of human normal and adjacent atherosclerotic plaque. Arteriosclerosis 6: 407–417
Wight TN (1989) Cell biology of arterial proteoglycans. Arteriosclerosis 9: 1–20
Wight TN (1995) The vascular extracellular matrix. In: Fuster V, Ross R, Topol E (eds) Atherosclerosis and coronary artery disease. Raven Press Ltd, New York
Williams KJ, Tabas I (1995) The response to retention hypothesis. Arterioscler Thromb 15: 551–561
Yao LY, Moody C, Schönherr E, Wight TN, Sandell LJ (1994) Identificational the proteoglycan versican in aorta and smooth muscle cells by DNA sequence analysis, in situ hybridization and immunocytochemistry. Matrix Biol 14: 213–225
Ylä Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T (1986) Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 54 (4): 402–407
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kruse, R., Merten, M., Buddecke, E. et al. Cholesterol-dependent changes of glycosaminoglycan pattern in human aorta. Basic Res Cardiol 91, 344–352 (1996). https://doi.org/10.1007/BF00788713
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00788713