Abstract
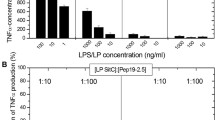
The investigational antitumour agent 5,6-dimethyl-xanthenone-4-acetic acid (5,6-MeXAA) induced dosedependent haemorrhagic necrosis of colon 38 tumours to a similar extent to that induced using bacterial lipopolysaccharide (LPS). TNF-α activity in serum and mRNA for TNF-α in splenocytes were induced over a broad range of LPS doses, whereas with 5,6-MeXAA, induction occurred only at concentrations approaching the maximum tolerated dose. At concentrations that provided similar degrees of haemorrhagic necrosis, the levels of serum TNF-α induced using 5,6-MeXAA were 100-fold lower than those obtained with LPS, indicating that haemorrhagic necrosis was not directly correlated with TNF-α levels. There was also no correlation between the degree of tumour necrosis and the duration of growth delay. Treatment with LPS did not induce a singificant delay in growth, despite extensive tumour haemorrhagic necrosis, whereas with 5,6-MeXAA, treatments that improved the cure rate did not necessarily give longer growth delays. Therapy using a combination of sub-optimal doses of both compounds was synergistic for the induction of serum TNF-α and message for TNF-α but was not synergistic for antitumour efficacy. Thus, no correlation is evident between cure rates, duration of growth delay, haemorrhagic necrosis and TNF-α induction by 5,6-MeXAA or LPS.
Similar content being viewed by others
References
Andersson J, Nagy S, Bjork L, Abrams J, Holm S, Andersson U (1992) Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev 127: 69–96
Atassi G, Briet P, Berthelon J-J, Collonges F (1985) Synthesis and antitumor activity of some 8-substituted 4-oxo-4H-1-benzopyrans. Eur J Med Chem 5: 393–402
Baguley BC, Calveley SB, Crowe KK, Fray LM, O'Rourke SA, Smith GP (1989) Comparison of the effects of flavone acetic acid, fostriecin, homoharringtonine and tumour necrosis factor α on colon 38 tumors in mice. Eur J Cancer Clin Oncol 25: 263–269
Berendt MJ, North RJ, Kirstein DP (1978) The immunological basis of endotoxin-induced tumour regression. Requirement for a pre-existing state of concomitant anti-tumour immunity. J Exp Med 148: 1560–1569
Bibby MC, Double JA, Loadman PM, Duke CV (1989) Reduction of tumor blood flow by flavone acetic acid: a possible component of therapy. J Natl Cancer Inst 81: 216–220
Bibby MC, Phillips RM, Double JA, Pratesi G (1991) Anti-tumour activity of flavone acetic acid (NSC-347512) in mice — influence of immune status. Br J Cancer 63: 57–62
Billiau A, Dijkmans R (1990) Interferon-gamma — mechanism of action and therapeutic potential. Biochem Pharmacol 40: 1433–1439
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 25: 3666–3670
Ching L-M, Baguley BC (1987) Induction of natural killer cell activity by the antitumour compound flavone acetic acid (NSC 347512). Eur J Cancer Clin Oncol 23: 1047–1050
Ching L-M, Baguley BC (1988) Enhancement of in vitro toxicity of mouse peritoneal exudate cells by flavone acetic acid (NSC 347512). Eur J Cancer Clin Oncol 24: 1521–1525
Ching L-M, Baguley BC (1989) Effect of flavone acetic acid (NSC 347512) on splenic cytotoxic effector cells and their role in tumour necrosis. Eur J Cancer Clin Oncol 25: 821–828
Ching L-M, Baguley BC (1989) Hyporesponsiveness of macrophages from C3H/HeJ (endotoxin-resistant) mice to the antitumour drug flavone acetic acid (NCS 347512). Eur J Cancer Clin Oncol 25: 1513–1515
Ching L-M, Joseph WR, Zhuang L, Atwell GJ, Rewcastle GR, Denny WA, Baguley BC (1991) Induction of natural killer activity by xanthenone analogues of flavone acetic acid: relation with antitumour activity. Eur J Cancer 27: 79–83
Ching L-M, Finlay GJ, Joseph WR, Baguley BC (1991) In vitro methods for screening agents with an indirect mechanism of antitumour activity: xanthenone analogues of flavone acetic acid. Eur J Cancer 27: 1684–1689
Ching L-M, Joseph WP, Baguley BC (1992) Stimulation of macrophage tumoricidal activity by 5,6-dimethyl-xanthenone-4-acetic acid a potent analogue of the antitumor agent flavone-8-acetic acid. Biochem Pharmacol 44: 192–195
Ching L-M, Joseph WR, Baguley BC (1992) Antitumour responses to flavone-8-acetic acid and 5,6-dimethylxanthenone-4-acetic acid in immune deficient mice. Br J Cancer 66: 128–130
Ching L-M, Joseph WR, Baguley BC (1993) Inhibition of antitumour effects of flavone acetic acid by cortisone. Anticancer Res 13: 1139–1141
Ching L-M, Joseph WR, Baguley BC (1993) Effect of tumour growth on the macrophage response to the antitumour agent 5,6-dimethylxanthenone-4-acetic acid. Anticancer Res 13: 2069–2075
Ching L-M, Joseph WR, Crosier KE, Baguley BC (1994) Induction of tumor necrosis factor-alpha messenger RNA in human and murine cells by the flavone acetic acid analogue 5,6-dimethylxanthenone-4-acetic acid (NSC 640488). Cancer Res 54: 870–872
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159
Chun M, Hoffman MK (1987) Combination immunotherapy of cancer in a mouse model: synergism between tumor necrosis factor and other defense systems. Cancer Res 47: 115–118
Damia G, Tagliabue G, Allavena P, D'Incalci M (1990) Flavone acetic acid antitumour activity against a mouse pancreatic adenocarcinoma is mediated by natural killer cells. Cancer Immunol Immunother 32: 241–244
Dong Z, Qi X, Fidler IJ (1993) Tyrosine phosphorylation of mitogen-activated protein kinases is necessary for activation of murine macrophages by natural and synthetic bacterial products. J Exp Med 177: 1071–1077
Engelhardt R, Mackensen A, Galanos C (1991) Phase-I trial of intravenously administered endotoxin (Salmonella abortus equi) in cancer patients. Cancer Res 51: 2524–2530
Finlay GJ, Wilson WR, Baguley BC (1986) Comparison of in vitro activity of cytotoxic drugs toward human carcinoma and leukaemia cell lines. Eur J Cancer Clin Oncol 22: 655–662
Futami H, Eader LA, Back TT, Gruys E, Young HA, Wiltrout RH, Baguley BC (1992) Cytokine induction and therapeutic synergy with interleukin-2 against murine renal and colon cancers by xanthenone-4-acetic acid derivatives. J Immunother 12: 247–255
Giavazzi R, Garofalo A, Damia G, Garatteni S, D'Incalci M (1988) Response to flavone acetic acid (NSC 347512) of primary and metastatic human colorectal carcinoma xenografts. Br J Cancer 57: 277–280
Hill S, Williams KB, Denekamp J (1989) Vascular collapse after flavone acetic acid. A possible mechanism of its antitumour action. Eur J Cancer Clin Oncol 25: 1419–1423
Hogan MM, Vogel SN (1990) Measurement of tumor necrosis factor α and β. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W (eds) Current protocols in immunology, vol 1. Greene Publishing Associates/Wiley Interscience, New York, p 6.10.1
Hornung RA, Back TC, Zaharto DS, Urba WJ, Longo DL, Wiltrout RH (1988) Augmentation of natural killer (NK) activity, induction of interferon and development of tumor immunity during the successful treatment of established murine renal cancer using flavone acetic acid (FAA) and interleukin 2. J Immunol 141: 3671–3679
Hornung RA, Young HA, Urba WJ, Wiltrout RH (1988) Immunomodulation of natural killer cell activity by flavone acetic acid, occurrence via induction of interferon α/β. J Natl Cancer Inst 80: 1226–1231
Kerr DJ, Kaye SB (1989) Flavone acetic acid — preclinical and clinical activity. Eur J Cancer Clin Oncol 25: 1271–1272
Lee JD, Kato K, Tobias PS, Kirkland TN, Ulevitch RJ (1992) Transfection of CD14 into 70Z/3 cells dramatically, enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med 175: 1697–1705
Lei M-G, Stimpson SA, Morrison DC (1991) Specific endotoxic lipopolysaccharide-binding receptors on murine splenocytes. III. Binding specificity and characterisation. J Immunol 147: 1925–1932
Mace KF, Hornung RL, Wiltrout RH, Young HA (1990) Correlation between in vivo induction of cytokine gene expression by flavone acetic acid and strict dose dependency and therapeutic efficacy against murine renal cancer. Cancer Res 50: 1742–1747
Mahadevan V, Malik STA, Meager A, Fiers W, Lewis GP, Hart IR (1990) Role of tumor necrosis factor in flavone acetic acid-induced tumor vasculature shutdown. Cancer Res 50: 5537–5542
McKeage MJ, Kestell P, Denny WA, Baguley BC (1991) Plasma pharmacokinetics of the antitumour agents 5,6-dimethylxanthenone-4-acetic acid, xanthenone-4-acetic acid and flavone-8-acetic acid in mice. Cancer Chemother Pharmacol 28: 409–413
Murray JC, Smith KA, Stern DM (1991) Flavone acetic acid potentiates the induction of endothelial procoagulant activity by tumour necrosis factor. Eur J Cancer 27: 765–770
Nakano K, Abe S, Sohmura Y (1986) Recombinant human tumor necrosis factor. I. Cytotoxic activity in vitro. Int J Immunopharmacol 8: 347–355
Plowman J, Naryanan VL, Dykes D, Szarvasi E, Briet P, Yoder OC, Paull KD (1986) Flavone acetic acid: a novel agent with preclinical antitumor activity against colon adenocarcinoma 38 in mice. Cancer Treat Rep 70: 631–638
Pratesi G, Rodolfo M, Rovetta G, Parmiani G (1990) Role of T cells and tumour necrosis factor in antitumour activity and toxicity of flavone acetic acid. Eur J Cancer 26: 1079–1083
Raetz CRH, Ulevitch RJ, Wright SD, Sibley CH, Ding AH, Nathan CF (1991) Gram-negative endotoxin — an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5: 2652–2660
Rewcastle GW, Kestell P, Baguley BC, Denny WA (1990) Light-induced breakdown of flavone acetic acid and xanthenone analogues in solution. J Natl Cancer Inst 82: 528–529
Rewcastle GW, Atwell GJ, Zhuang L, Baguley BC, Denny WA (1991) Potential antitumor agents. 61. Structure-activity relationships for in vivo colon-38 activity among disubstituted 9-oxo-9H-xanthene-4-acetic acids. J Med Chem 34: 217–222
Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ (1990) Structure and function of lipopolysaccharide binding protein. Science 249: 1429–1431
Smith GP, Calveley SB, Smith MJ, Baguley BC (1987) Flavone acetic acid (NSC 347512) induces haemorrhagic necrosis of mouse colon 26 and 38 tumours. Eur J Cancer Clin Oncol 23: 1209–1212
Sugarman BJ, Aggarwal BB, Hass PE, Figar IS, Palladino MA Jr, Shepard HM (1985) Recombinant human tumor necrosis factor-α effects on proliferation of normal and transformed cells in vitro. Science 230: 943–945
Thomsen LL, Ching L-M, Baguley BC (1990) Evidence for the production of nitric oxide by activated macrophages treated with the antitumor agents flavone-8-acetic acid and xanthenone-4-acetic acid. Cancer Res 50: 6966–6970
Weinstein SL, Gold MR, Defranco AL (1991) Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci USA 88: 4148–4152
Wiltrout RH, Boyd MR, Back TC, Salup RR, Arthur JA, Hornung RL (1988) Flavone-8-acetic acid augments systemic natural killer cell activity and synergizes with IL-2 for treatment of murine renal cancer. J Immunol 140: 3261–3265
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433
Zwi LJ, Baguley BC, Gavin JB, Wilson WR (1989) Blood flow failure as a major determinant in the antitumor action of flavone acetic acid (NSC 347512). J Natl Cancer Inst 81: 1005–1012
Zwi LJ, Baguley BC, Gavin JB, Wilson WR (1990) The use of vascularised spheroids to investigate the action of flavone acetic acid on tumour blood vessels. Br J Cancer 62: 231–237
Zwi LJ, Baguley BC, Gavin JB, Wilson WR (1994) Correlation between immune and vascular activities of xanthenone acetic acid antitumor agents. Oncol Res 6: 79–85
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ching, LM., Joseph, W.R., Zhuang, L. et al. Interaction between endotoxin and the antitumour agent 5,6-dimethylxanthenone-4-acetic acid in the induction of tumour necrosis factor and haemorrhagic necrosis of colon 38 tumours. Cancer Chemother. Pharmacol. 35, 153–160 (1994). https://doi.org/10.1007/BF00686639
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686639