Summary
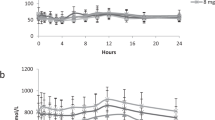
The metabolism of amitriptyline (AMT) has been studied in two groups of depressed in-patients on long term AMT therapy: 11 patients with no other major disease and 8 patients with chronic renal failure, who were being dialysed. The patients with renal insufficiency had decreased concentrations of AMT, nortriptyline (NT) and their unconjugated hydroxymetabolites compared to patients with normal kidney function. The plasma levels of conjugated products were extremely high in the uraemics. The latter metabolites are probably inert. The reduced concentration of unconjugated hydroxymetabolites, which are active compounds, may decrease the clinical effectiveness of the drug.
Similar content being viewed by others
Abbreviations
- AMT:
-
amitriptyline
- OHAMT:
-
hydroxyamitriptyline
- NT:
-
nortriptyline
- OHNT:
-
hydroxynortriptyline
- -c:
-
conjugated
- Nc:
-
non conjugated
- RF:
-
renal failure
References
Alexanderson B, Borgå O (1973) Urinary excretion of nortriptyline and five of its metabolites in man after single and multiple oral dose. Eur J Clin Pharmacol 5: 174–180
Åsberg M, Sjöqvist F (1981) Therapeutic monitoring of tricyclic antidepressants. Clinical aspects. In: Therapeutic drug monitoring (eds) A. Richens, V. Marks, London, Churchill Livingstone, 224–238
Baun de JR, Miller EC, Miller JA (1970) N-hydroxy-2-acetyl-aminofluorene sulfotransferase: its probable role in carcinogenesis and protein. (methion-S-yl) binding in rat liver. Cancer Res 30: 577–595
Bennett WM, Singer I, Golper T, Feig P, Coggins CJ (1977). Guidelines for drug therapy in renal failure. Ann Intern Med 86: 754–783
Bertilsson L, Eichelbaum M, Mellström B, Säwe J, Schulz HU, Sjöqvist F (1980) Nortriptyline and antipyrine clearance in relation to debrisoquine hydroxylation in man. Life Sci 27: 1673–1677
Bertilsson L, Mellström B, Säwe J, Sjöqvist F (1982) Pharmacogenetic aspects on the metabolism of tricyclic antidepressants. In: Langer SZ et al. (eds) Advances in the biosciences, vol 40. New vistas in depression. Pergamon Press, Oxford New York
Bertilsson L, Mellström B, Sjöqvist F (1979) Pronounced inhibition of noradrenaline uptake by 10-hydroxy-metabolites of nortriptyline. Life Sci 25: 1285–1292
Bianchetti G, Graziani G, Brancaccio D, Morganti A, Leonetti G, Manfrin M, Sega R, Gomeni R, Ponticelli C, Morselli PL (1976) Pharmacokinetics and effects of propranolol in terminal uremic patients and in patients undergoing regular dialysis treatment. Clin Pharmacokinet 1: 373–384
Biggs SR, Chasseaud LF, Hawkins DR, Midgley I (1979) Determination of amitriptyline and its major basic metabolites in human urine by high-performance liquid chromatography. Drug Metab Dispos 7 [4] 233–236
Borgå O, Hoppel C, Odar-Cederlöf I (1979) Plasma levels and renal excretion of phenytoin and its metabolites in patients with renal failure. Clin Pharmacol Ther 26 [3] 306–314
Braithwaite RA (1976) The significance of drug interactions in the evaluation of psychotropic drugs. Br J Clin Pharmacol [Suppl]: 29–34
Caddy B, Fish F, Tranter J (1976) Studies on the oxidation of amitriptyline. Analyst 101: 244–254
Crammand WA, Knight PR, Lawrence JR, Higgins BA, Court JH, McNemara FM, Clarkson AR, Miller CDJ (1968) Psychological aspects of the management of chronic renal failure. Br Med J 1: 539–543
Dawling S, Lynn K, Rosser R, Braithwaite R (1981) The pharmacokinetics of nortriptyline in patients with renal failure. Br J Clin Pharmacol 12: 39–45
Dawling S, Lynn K, Rosser R, Braithwaite R (1982) Nortriptyline metabolism in chronic renal failure: metabolite elimination. Clin Pharmacol Ther 32 [3] 322–329
Eschenhof E, Rieder J (1969) Untersuchungen über das Schicksal des Antidepressivums Amitriptylin im Organismus der Ratte und des Menschen. Arzneimittelforsch 19: 957–966
Faed EM (1980) Decreased clearance of diflunisal in renal insufficiency. An alternative explanation. Br J Clin Pharmacol 10: 185–186
Farmer CJ, Snowden SA, Parsons V (1979) The prevalence of psychiatric illness among patients on home haemodialysis. Psychol Med 9: 509–514
Fisher LJ, Thies RL, Chrakowski D, Donham KJ (1980) Formation and urinary excretion of cyproheptadine glucuronide in monkey, chimpanzees, and human. Drug Metab Dispos 8 [6]: 422–424
Gram LF, Overø KF, Kirk L (1974) Influence of neuroleptics and benzodiazepines on metabolism of tricyclic antidepressants in man. Am J Psychiatr 131: 863
Gugler R, Kürten JW, Jensen CJ, Klehr U, Hartlapp J (1979) Clofibrate disposition in renal failure and acute and chronic liver disease. Eur J Clin Pharmacol 15: 341–347
Gurney C, Roth M, Garside RF, Kerr TA, Schapira K (1972) Studies in the classification of affective disorders. Br J Psychiatr 121: 162
Heikkila RE, Goldfinger SS, Orlansky H (1976) The effect of various phenothiazines and tricyclic antidepressants on the accumulation of (3H)-norepinephrine and (3H)-5-hydroxytryptamine in slices of rat occipital cortex. Res Commun Chem Pathol Pharmacol 13: 237–250
Hucker HB, Stauffer SC, Balleto AJ, White SD, Zacchei AG, Arison BH (1978) Physiological disposition and metabolism of cyclobenzaprine in the rat, dog, rhesus monkey and man. Drug Metab Dispos 6 [6] 659–672
Javid JI, Perel JM, Davis JM (1979) Inhibition of biogenic amines uptake by imipramine, desimipramine, 2-OH-imipramine and 2-OH-desimipramine in rat brain. Life Sci 24: 21–28
Kampf D, Roots I, Hildebrandt AG (1980) Urinary excretion of D-glucaric acid, an indicator of drug metabolizing enzyme activity, in patients with impaired renal function. Eur J Clin Pharmacol 18: 255–261
Kober A, Sjöholm I, Borgå O, Odar-Cederlöf (1979) Protein binding of diazepam and digitoxin in uremic and normal serum. Biochem Pharmacol 28: 1037–1042
Krijgsheld KR, Koster HJ, Scholtens E, Mulder GJ (1982) Cholestatic effect of harmol glucuronide in the rat. Prevention of harmol induced cholestasis by increased formation of harmol sulfate. J Pharmacol Exp Ther 221 [3] 731–734
Levy NB (1978) Coping with maintenance haemodialysis. Psychological considerations in the care of patients. In: Massry SG, Sellers AL (eds) Clinical aspects of uraemia and haemodialysis. Thomas, Springfield, III, USA
Levy G (1979) Decreased body clearance of diflunisal in renal insufficiency. An alternative explanation. Br J Clin Pharmacol 8: 601
Lowenthal DT, Øie S, Van Stone JC, Briggs WA, Levy G (1976) Pharmacokinetics of acetaminophen elimination by anephric patients. J Pharmacol Exp Ther 196 [3] 570–578
Mc Cormick M, Navarro V (1973) Prevalence of chronic renal failure and access to dialysis. Int J Epidemiol 2: 247–255
Meerman JHN, Mulder GJ (1981) Prevention of the hepatotoxic action of N-hydroxy-2-acetylaminofluorene in the rat by inhibition of N-O-sulfation by pentachlorophenol. Life Sci 28: 2361–2365
Mellström B, Bertilsson L, Säwe J, Schulz HU, Sjöqvist F (1981) E and Z 10-hydroxylation of nortriptyline: relationship to polymorphic debrisoquine hydroxylation. Clin Pharmacol Ther 30 [2] 189–193
Meyers M, Slikker W, Pascoe G, Vore M (1980) Characterization of cholestasis induced by estradiol-17B-D-glucuronide in the rat. J Pharmacol Exp Ther 214: 87–93
Nemeth I, Szeleczki T (1981) Enhanced drug metabolism and renal dysfunction. Br J Clin Pharmacol 11: 92–93
Odar-Cederlöf I, Borgå O (1974) Kinetics of diphenylhydantoin in uremic patients: consequences of decreased plasma protein binding. Eur J Clin Pharmacol 7: 31–37
Odar-Cederlöf I, Vessman J, Alvan G, Sjöqvist F (1977) Oxazepam disposition in uremic patients. Acta Pharmacol Toxicology [Suppl 1] 40: 52–62
Piafsky KM, Borgå O, Odar-Cederlöf I, Johansson C, Sjöqvist F (1978) Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma α1 acid glycoprotein. N Engl J Med 299: 1435–1439
Potter WZ, Calil HM, Manian AA, Zavadil AP, Goodwin FK (1979) Hydroxylated metabolites of tricyclic antidepressants: preclinical assessment of activity. Biol Psychiatry 14 [4]: 601–613
Reidenberg MM (1977) The binding of drugs to plasma proteins and the interpretation of measurement of plasma concentrations of drugs in patients with poor renal function. Am J Med 62: 466–470
Reidenberg MM (1977) The biotransformation of drugs in renal failure. Am J Med 62: 482–485
Reidenberg MM, Odar-Cederlöf I, Bahr von C, Borgå O, Sjöqvist F (1971) Protein binding of diphenylhydantoin and desmethylimipramine in plasma from patients with poor renal function. N Engl J Med 264–267
Rollins DE, Klaassen CD (1979) Biliary excretion of drugs in man. Clin Pharmacokinet 4: 368–379
Rosser R (1976) Depression during renal dialysis and following transplantation. Proc R Soc Med 69: 832–834
Sandoz M, Vandel S, Vandel B, Bonin B, Allers G, Volmat R (1983) Biotransformation of amitriptyline in alcoholic depressive patients. Eur J Clin Pharmacol 24: 615–621
Sellman R, Kanto J, Raijola E, Pekkarinen (1975) Induction effect of diazepam on its own metabolism. Acta Pharmacol Toxicol 37: 345–351
Sjöqvist F, Berglund F, Borgå O, Hammer W, Andersson S, Thorstrand C (1969) The pH dependent excretion of monomethylated tricyclic antidepressant in dog and man. Clin Pharmacol Ther 10: 826–833
Stevenson IH, Browning M, Crooks J (1972) Changes in human drug metabolism after long term exposure to hypnotics. Br Med J 4: 322–324
Tillement JP, Lhoste F, Giudicelli JF (1978) Diseases and drug protein binding. Clin Pharmacokinet 3: 144–154
Vandel B, Sandoz M, Vandel S, Allers G, Volmat R (1982) Biotransformation of amitriptyline in depressive patients — urinary excretion of seven metabolites. Eur J Clin Pharmacol 22 [3] 239–245
Vandel B, Vandel S, Allers G, Bechtel P, Volmat R (1979) Interaction between amitriptyline and phenothiazine in man: effect on plasma concentration of amitriptyline and its metabolite nortriptyline and the correlation with clinical response. Psychopharmacology 65: 187–190
Vandel S, Vandel B, Sandoz M, Allers G, Bechtel P, Volmat R (1978) Clinical response and plasma concentration of amitriptyline and its metabolite nortriptyline. Eur J Clin Pharmacol 14: 185–190
Verbeeck RK (1982) Glucuronidation and disposition of drug glucuronides in patients with renal failure. A review. Drug Metab Dispos 10 [1] 87–89
Verbeeck RK, Branch RA, Wilkinson GR (1981) Drug metabolites in renal failure: Pharmacokinetic and clinical implications. Clin Pharmacokinet 6: 329–345
Verbeeck R, Tjandramaga TB, Mullie A, Verbesselt R, Verberckmoes R, De Schepper PJ (1979) Biotransformation of diflunisal and renal excretion of its glucuronides in renal insufficiency. Br J Clin Pharmacol 7: 273–282
Verbeeck R, Tjandramaga TB, Verberckmoes R, De Schepper PJ (1976) Biotransformation and excretion of lorazepam in patients with chronic renal failure. Br J Clin Pharmacol 3: 1033–1039
Walle Th, Conradi EC, Walle UK, Fagan TC, Gaffney TE (1979) Propranolol glucuronide cumulation during long-term propranolol therapy: a proposed storage mechanism for propranolol. Clin Pharmacol Ther 26 [6]: 686–695
Watabe T, Ishizuka T, Isobe M, Ozawa N (1982) A 7-hydroxymethyl sulfate ester as an active metabolite of 7,12-dimethylbenz (a) anthracene. Science 215: 403–404
Yousef IM, Tuchweber B, Vonk RJ, Masse D, Audet M, Roy CC (1981) Lithocholate cholestasis-sulfated glycolithocholate induced intrahepatic cholestasis in rats. Gastroenterology 80: 233–241
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sandoz, M., Vandel, S., Vandel, B. et al. Metabolism of amitriptyline in patients with chronic renal failure. Eur J Clin Pharmacol 26, 227–232 (1984). https://doi.org/10.1007/BF00630290
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00630290