Summary
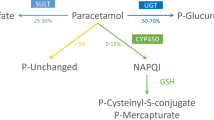
A simple, noninvasive procedure was developed to monitor glucuronidation and sulphation in patients using paracetamol as the test drug. Urinary paracetamol and its metabolites were determined by UV absorption and electrochemical detection after separation by HPLC. The metabolite to paracetamol ratio (M/P) was used as an approximation of the partial clearance due to metabolite formation. In 14 healthy volunteers, all nonsmokers without medication, M/P was 18±5 for glucuronides and 12±4 for sulphate esters. The test was validated in patients treated with enzyme inducers. In 10 patients with epilepsy given phenytoin 0.3 g/day, and in 10 patients with tuberculosis treated with rifampicin 0.6 g/day, the M/P value for glucuronidation was significantly increased to 41±11 and 35±7, respectively. In contrast, M/P values for sulphation were not significantly different from untreated controls. In 9 heavy smokers (about 40 cigarettes/day) M/P values for glucuronidation were also significantly increased to 33±11. However, in 4 moderate smokers (about 10 cigarettes/day) no significant increase was found. The results suggest that in man glucuronidation of paracetamol is inducible both by phenobarbital-and 3-methylcholanthrene-type inducers. Monitoring the ratios of various urinary paracetamol conjugates/paracetamol may be useful as a new tool for the evaluation of factors determining glucuronide and sulphate ester formation in man.
Similar content being viewed by others
References
Dutton GJ (1980) Glucuronidation of drugs and other compounds. CRC Press, Boca Raton, Florida
Josting D, Winne D, Bock KW (1976) Glucuronidation of paracetamol, morphine and 1-naphthol in the rat intestinal loop. Biochem Pharmacol 25: 613–616
Bock KW, von Clausbruch UC, Kaufmann R, Lilienblum W, Oesch F, Pfeil H, Platt KL (1980) Functional heterogeneity of UDP-glucuronosyltransferase in rat tissues. Biochem Pharmacol 29: 495–500
Witassek F, Bircher J, Huguenin P, Preisig R (1983) Abnormal glucuronidation of zomepirac in patients with cirrhosis of the liver. Hepatology 3: 415–422
Shull HJ Jr, Wilkinson GR, Johnson R, Schenker S (1976) Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Int Med 84: 420–425
Kraus JW, Desmond PV, Marshall JP, Johnson BF, Schenker S, Wilkinson GR (1978) Effect of age and liver disease on disposition of lorazepam. Clin Pharmacol Ther 24: 411–419
Patwardhan RV, Johnson RF, Hoyumpa A Jr, Sheehan A, Desmond PV, Wilkinson GR, Branch RA, Schenker S (1981) Normal metabolism of morphine in cirrhosis. Gastroenterol 81: 1006–1011
Arias IM, Gartner LM, Cohen M, Ezzer JB, Levi AJ (1969) Chronic nonhemolytic unconjugated hyperbilirubinemia with glucuronyl transferase deficiency. Am J Med 47: 395–398
Levy G, Yamada H (1971) Drug biotransformation interactions in man III: Acetaminophen and salicylamide. J Pharm Sci 60: 215–221
Prescott LF (1980) Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 10: 291S-298S
Miners JO, Attwood J, Birkett DJ (1984) Determinations of acetaminophen metabolism: Effect of inducers and inhibitors of drug metabolism on acetaminophen's metabolic pathways. Clin Pharmacol Ther 35: 480–486
Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB (1973) Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187: 185–195
Howie D, Adriaenssens PI, Prescott LF (1977) Paracetamol metabolism following overdosage: Application of high performance liquid chromatography. J Clin Pharmacol 29: 235–237
Moldeus P (1978) Paracetamol metabolism and toxicity in isolated hepatocytes from rat and mouse. Biochem Pharmacol 27: 2859–2863
Lustgarten JA, Wenk RE (1972) Simple, rapid kinetic method for serum creatinine measurement. Clin Chem 18: 1419–1422
Ohnhaus EE, Park BK (1979) Measurement of urinary 6-β-hydroxycortisol excretion as an in vivo parameter in the clinical assessment of the microsomal enzyme-inducing capacity of antipyrine, phenobarbitone and rifampicin. Eur J Clin Pharmacol 15: 139–145
Holm St (1979) A simple sequentially rejective multiple test procedure. Scand Statist 6: 65–70
Prescott LF, Wright N (1973) The effects of hepatic and renal damage on paracetamol metabolism and excretion following overdose. Br J Pharmacol 49: 602–613
Perucca E, Richens A (1979) Paracetamol disposition in normal subjects and in patients treated with antiepileptic drugs. Br J Clin Pharmacol 7: 201–206
Prescott LF, Critchley JAJH, Balali-Mood M, Pentland B (1981) Effects of microsomal enzyme induction on paracetamol metabolism in man. Br J Clin Pharmacol 12: 149–153
Mucklow JC, Fraser HS, Bulpitt CJ, Kahn C, Mould G, Dollery CT (1980) Environmental factors affecting paracetamol metabolism in London factory and office workers. Br J Clin Pharmacol 10: 67–74
Price Evans DA, Mahgoub A, Sloan TP, Idle JR, Smith RL (1980) A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet 17: 102–105
Meier PJ, Mueller HK, Dick B, Meyer UA (1983) Hepatic monooxygenase activities in subjects with a genetic defect in drug oxidation. Gastroenterology 85: 682–692
Nash RM, Stein L, Penno MB, Passananti GT, Vesell ES (1984) Sources of interindividual variations in acetaminophen and antipyrine metabolism. Clin Pharmacol Ther 36: 417–430
Pantuck EJ, Pantuck CB, Anderson KE, Wattenberg LW, Conney AH, Kappas A (1984) Effect of Brussels sprouts and cabbage on drug conjugation. Clin Pharmacol Ther 35: 161–169
Loub WD, Wattenberg LW, Davis DW (1975) Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst 54: 985–988
Ochs HR, Otten H (1981) Einfluß von Alter, Geschlecht und Rauchgewohnheiten auf die Kinetik von Oxazepam. Verh Dtsch Ges Inn Med 78: 1205–1208
Seideman P, Ericsson Ö, Gröningsson K, von Bahr C (1981) Effect of pentobarbital on the formation of diastereomeric oxazepam glucuronides in man: analysis by high performance liquid chromatography. Acta Pharmacol Toxicol 49: 200–204
Fleischmann R, Stärz U, Remmer H (1985) Increased activity of certain isoenzymes of cytochrome P-450 and UDP-glucuronyltransferases in the liver of cigarette smokers. In: Matern S, Bock KW, Gerok W (eds) Advances in glucuronide conjugation. MTP Press, Lancaster, pp 351–365
Bock KW, Josting D, Lilienblum W, Pfeil H (1979) Purification of rat liver microsomal UDP-glucuronyltransferase. Eur J Biochem 98: 19–26
Falany CN, Tephly TR (1983) Separation, purification and characterization of three isoenzymes of UDP-glucuronyltransferase from rat liver microsomes. Arch Biochem Biophys 227: 248–258
Blanckaert N, Schmid R (1982) Physiology and pathophysiology of bilirubin metabolism. In: Zakim D, Boyer TD (eds) Hepatology. A textbook of liver disease. WB Saunders, pp 246–296
Roy Chowdhury J, Roy Chowdhury N, Falany CN, Tephly TR, Arias IM (1986) Isolation and characterization of multiple forms of rat liver UDP-glucuronate glucuronosyltransferase. Biochem J 233: 827–837
Bock KW, Fröhling W, Remmer H, Rexer B (1973) Effects of phenobarbital and 3-methylcholanthrene on substrate specificity of rat liver microsomal UDP-glucuronyltransferase. Biochim Biophys Acta 327: 46–56
Wishart GJ (1978) Demonstration of functional heterogeneity of hepatic uridine diphosphate glucuronosyltransferase activities after administration of 3-methylcholanthrene and phenobarbital to rats. Biochem J 174: 671–672
Watkins JB, Gregus Z, Thompson TN, Klaassen CD (1982) Induction studies on the functional heterogeneity of rat liver UDP-glucuronosyltransferase. Toxicol Appl Pharmacol 64: 439–446
Bock KW, Lilienblum W, von Bahr C (1984) Studies of UDP-glucuronyltransferase activities in human liver microsomes. Drug Metab Dispos 12: 93–97
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bock, K.W., Wiltfang, J., Blume, R. et al. Paracetamol as a test drug to determine glucuronide formation in man. Effects of inducers and of smoking. Eur J Clin Pharmacol 31, 677–683 (1987). https://doi.org/10.1007/BF00541295
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00541295